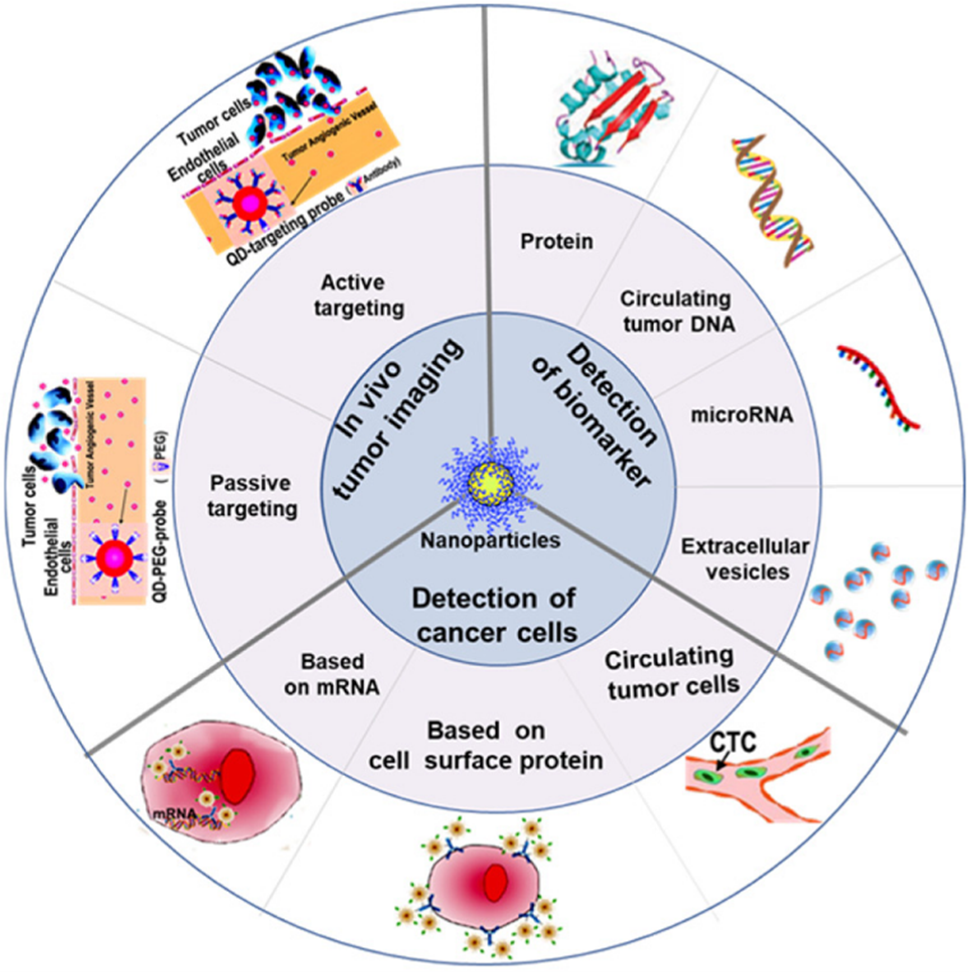
Sensing In Vitro
The discovery of cancer-associated molecular alterations has led to new opportunities for early cancer detection. These alterations are found in the blood by employing genomic, epigenomic, transcriptomic, proteomic, and metabolomic assays. Nanotechnology-enabled liquid biopsy diagnostic devices make it possible to enrich for blood-circulating ‘cancer fingerprints’ and then perform omics analyses. Through well-established fabrication techniques, such as lithography, compact devices or point-of-care systems are crafted. These diagnostic tools use biochemical reactions to interrogate the presence, activity, or concentration of specific biological molecules in a bodily fluid sample. These reactions, such as antigen-antibody binding, hybridization of DNA fragments, or ligand adhering to cell surface epitopes, can subsequently be transformed into measurable detection signals using methods like optical, magnetic, or electronic means.
Detection and diagnostic devices using nanoparticles have been developed for many cancers (see the table below). In a pioneering study (Jwa-min Nam, Shad Thaxton, and Chad A. Mirkin, Science, 2003), an ultrasensitive method for detecting prostate-specific antigen (PSA) was developed. It detected PSA down to 3 attomolar sensitivity level. This is six orders of magnitude more sensitive compared to clinically accepted conventional assays. This approach, known as the bio-barcode assay, employs a sandwich immunoassay design that combines magnetic nanoparticles with PSA-specific antibodies and DNA-encoded gold nanoparticles. The elevated PSA level triggers prostate-specific membrane antigen (PSMA) targeted PET imaging (Steven P. Rowe, Michael A. Gorin, and Martin G. Pomper, Annual Review of Medicine, 2019). This is useful for prostate cancer detection and therapy monitoring. Another sandwich immunoassay device has been designed, relying on DNA-encoded antibody libraries (DEAL, as shown in the right figure). The DEAL technique (Rong Fan, et al., Nature Biotechnology, 2008; Chao Ma, et al., Nature Methods, 2011) uses DNA-directed immobilization of antibodies in microfluidic channels. This can convert a pre-patterned single stranded DNA barcode microarray into an antibody microarray. This assay can be used in multiplex immunoassays, on-chip blood separation, and measurement of large protein panels directly from blood. Looking forward, new advances in the coupling of complex microfluidics and nanoscale materials offer many benefits. They have the potential to gain comprehensive in vitro cancer diagnostic insights from a patient sample.
Examples of nanomaterial detection platforms used in cancer liquid biopsy studies. EpCAM, epithelial cell adhesion molecule. Adapted from Lois Gardner, Kostas Kostarelos, Parag Mallick, Caroline Dive, and Marilena Hadjidemetriou, Nature Reviews Clinical Oncology, 2022.
| Nanomaterial platform | Enrichment mechanism | Biofluid (volume) | Type of cancer | Reference |
|---|---|---|---|---|
| Nanotrap hydrogel core–shell nanoparticles | Affinity capture | Serum (0.2 ml) | Ovarian or prostate cancer | Fredolini, C. et al. AAPS J. 2010 |
| PEGylated liposomal doxorubicin | Protein corona | Plasma (1 ml) | Ovarian cancer | Hadjidemetriou, M. et al. Nano Today 2020 |
| Proteograph superparamagnetic iron oxide nanoparticles | Protein corona | Plasma (0.1 ml) | Non-small-cell lung cancer | Blume, J. E. et al. Nat. Commun. 2020 |
| Nanomaterial platform | Enrichment mechanism | Biofluid (volume) | Type of cancer | Reference |
|---|---|---|---|---|
| GMACS chip (silica magnetic nanoparticles) | Label free | Serum (0.5 ml) | Breast cancer | Gwak, H. et al. Biomicrofluidics 2019 |
| Polypyrrole-coated gold nanowires | Label free | Plasma (0.2-1 ml) | Breast or lung cancer | Jeon, S. H. et al. Theranostics 2016 |
| Nanomaterial platform | Enrichment mechanism | Biofluid (volume) | Type of cancer | Reference |
|---|---|---|---|---|
| Magnetic nanowires | Immunocapture (anti-pCAM, anti-EGFR, anti-N-cadherin, anti-TROP2 and anti-vimentin) | Blood (0.2-1 ml) | Non-metastatic, early-stage breast cancer | Hong, W. et al. Biomaterials 2016 |
| TiO2 nanofibers | Immunocapture (anti-EpCAM) | Blood (1 ml) | Colorectal or gastric cancer | Zhang, N. et al. Adv. Mater.2012 |
| PL–PEG–NH2- functionalized graphene oxide nanosheets | Immunocapture (anti-EpCAM) | Blood (1 ml) | Lung, breast, or pancreatic cancer | Yoon, H. J. et al. Nat. Nanotechnol. 2013 |
| Carbon nanotube chip | Label free | Blood (4-8.5 ml) | Breast cancer | Loeian, M. S. et al. Lab. Chip 2019 |
| Nanomaterial platform | Enrichment mechanism | Biofluid (volume) | Type of cancer | Reference |
|---|---|---|---|---|
| Nano-interfaced microfluidic exosome chip (graphene oxide–polydopamine coated) | Immunocapture (anti-CD81) | Plasma (2 μl) | Ovarian cancer | Zhang, P. et al. Lab. Chip 2016 |
| Silicon nanowires | Immunocapture (anti-EpCAM, anti-ASGPR1 and anti-CD147) | Plasma (0.5 ml) | Hepatocellular carcinoma | Sun, N. et al. Nat. Commun.2020 |
Imaging in Vivo
The sensitivity of current imaging methods, such as MRI, PET, and CT, can only detect cancers once thousands of cancer cells have grown. By this time, the cancer may have spread. Nanotechnology-based imaging contrast agents are being developed and translated for conventional imaging modalities. These offer the ability to specifically target and significantly amplify the detection of tumors in vivo. Current nanoscale imaging platforms enables novel imaging modalities that are not traditionally used for clinical cancer treatment and diagnosis. Examples include photoacoustic tomography (PAT), Raman spectroscopic imaging, and multimodal imaging (i.e., contrast agents designed for several imaging modalities at the same time). Nanotechnology makes these sensitive imaging platforms possible. Nanoparticles are designed to carry multiple components, such as conventional contrast agents and ligands, to target tumor cells.
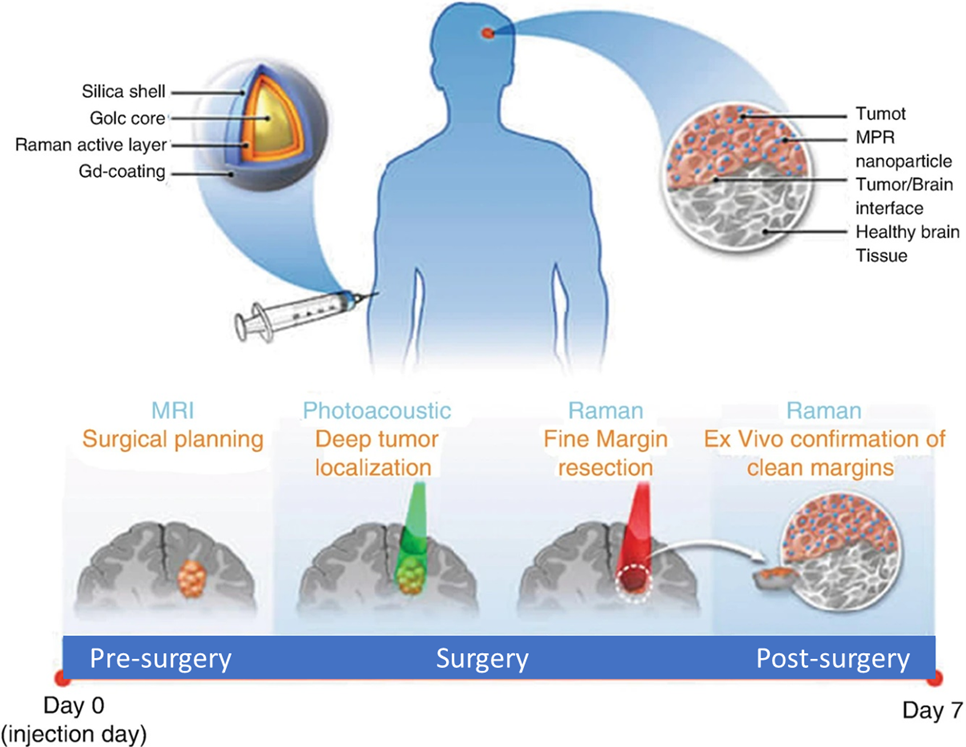
NCI-funded research has produced many notable nanotechnology-based imaging agents over the past decade. For instance, researchers have engineered multimodal nanoparticles that can delineate the margins of brain tumors before and during surgery (Moritz F Kircher, et al., Nature Methods, 2012). These nanoparticles, used in combination of MRI, PAT, and Raman detection, are capable of tracking tumor growth and surgical staging via MRI. These agents also enable the surgeon to visualize a brain tumor down to the single cancer cell level, increasing the potential to precisely remove cancerous tissue (see the figure below). Similarly, scientists have designed silica-hybrid nanoparticles (‘C-dots’). These nanoparticles can be used as contrast agents in PET and optical imaging in the same platform (Evan Philips, et al., Science Trans Med., 2016). They have been proven to actively target metastatic melanoma with cRGDY peptides in clinical trials.
Cancer nanotechnology has long been viewed as a way to simultaneously image and administer therapy in vivo. This has led to the emergence of ‘theranostic’ nanoscale platforms. A diagnostic platform for pancreatic cancer has been designed to break through the fibrotic stromal tissue of these tumors (Lei Zhu, Hui Mao, and Lily Yang, WIREs Nanomedicine and Nanobiotechnology, 2022). These nanoparticles contain magnetic iron cores. Once they are past the fibrotic barrier, they facilitate MRI contrast for diagnosis while they deliver small-molecule therapeutic drugs directly to cancer cells.
Measuring Response to Therapy
The cornerstone of precise and prognostic medical care lies in measuring an individual patient’s response to targeted therapy, immunotherapy, or surgery as their disease progresses. Accurate and relevant monitoring of disease progress can allow providers to optimize treatment regimens. Treatment can be optimized through therapeutic course correction, use of drug combinations, and fine-tuning drug doses. Prognostic methods are essential for preemptive clinical decision making, such as separating patients who respond to therapy from those who do not. It is also used to stratify patients for clinical trials. In vivo imaging, tissue biopsy, and in vitro diagnostics are suited to this purpose. It is important for oncologists to be able to track cancer cell death in vivo. It enables them to deliver effective dosing regimens and/or precisely administer novel therapies. For example, scientists have designed a target-enabled in situ ligand assembly (TESLA) nanoparticle system. In this system, nanoparticles can self-assemble within the body when cleaved by enzymes from therapy-induced cell death (Deju Ye, et al., Nature Chemistry, 2014). These nanoparticles also carry imaging contrast agents to monitor local tumor response to therapies.
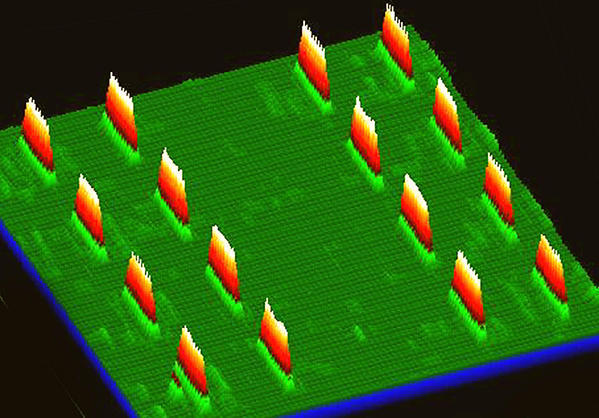
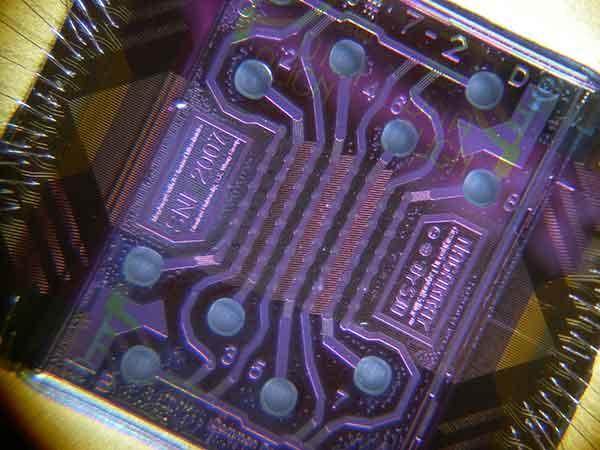
Tissue biopsy serves as the traditional gold standard for cancer identification. However, it is invasive and has limited space sampling capability for tracking disease progression relative to the course of therapy. In vitro cancer diagnosis and monitoring is a simpler method of tracking therapy response. Over time, it analyzes biological material shed by the tumor that circulates in blood. Cells, DNA, other cancer-specific biomolecules are detectable using these methods. There are incredibly low concentrations of these biomolecules compared to other blood components. Specific and highly sensitive tools are required to detect, capture, and purify them. Nanotechnology is making these essential tools possible. For instance, microfluidic nano chips (see figure at right) have been developed for multiplex protein detection of potential cancer markers at sub-picomolar concentrations (Sebastian J. Osterfeld, et, al., PNAS, 2008). They are also used for CTC detection, with over 95% sensitivity (Millicent Lin, et al., Accounts of Chemical Research, 2014). This new technology makes it possible to effectively monitor cells and proteins in blood samples. They help us enhance care and improve therapy outcomes.