Network Purpose
The network’s purpose is to provide comprehensive immune profiling of clinical trial specimens to identify biomarkers for improving immunotherapy, including improved selection of cancer patients for immunotherapy.
The CIMACs-CIDC Network, which is supported by multiple cooperative agreements, provides multimodal assays and correlative analysis to identify biomarkers of response, resistance, and toxicity to cancer immunotherapy. It aims to understand the mechanisms underlying these processes.
CIMACs work with clinical trial investigators on correlative studies across multiple cancer trials of immunotherapy. They use a range of harmonized and validated state-of-the-art assays, spanning genomics, proteomics, transcriptomics, immune phenotyping, functional analyses, and epigenetics.
Correlative study teams comprising CIMACs and clinical trial investigators send biomarker and clinical data, respectively, from the trials to a central database (CIDC). The teams then work with these data on both trial-specific and cross-trial analyses for biomarker discovery. Data from this centralized immuno-oncology (IO) database are also shared with the research community through the NCI Cancer Research Data Commons (CRDC).
Using the approaches listed below, the network addresses several challenges in IO cancer biomarker research, including the complexity of the tumor-immune interface, heterogeneity of methods and specimens across studies, and small trial sizes:
| Challenge | Approach |
|---|---|
| Complex interplay between cancer and immune system | Comprehensive specimen profiling of tumor, tumor microenvironment, blood, and other specimens |
| Variability in assay performance among labs | Harmonized and standardized state-of-the-art, multimodal assays Centralized bioinformatics pipelines |
| Tumor and interpatient heterogeneity | Standardized clinical data, specimens, and assays to enable cross-trial analysis |
| Small size of patient cohorts in clinical trials | Larger sample sizes through cross-trial analysis Central database of biomarker and clinical data from multiple trials |
Network Structure
An overview of the CIMACs-CIDC Network is published in Clinical Cancer Research.
CIMACs
The following four CIMACs are academic laboratories at leading cancer research institutions that perform multi-modal assay analysis using specimens from immunotherapy clinical trials:
- Dana-Farber Cancer Institute
- Icahn School of Medicine at Mount Sinai
- MD Anderson Cancer Center
- Stanford University
CIDC
NCI operates the data center, which collects clinical and biomarker data from the immunotherapy trials collaborating with CIMACs. CIDC performs the following:
- Distributes data to authorized researchers in the network.
- Standardizes assay data across trials using centralized bioinformatics pipelines.
- Intakes a standardized set of clinical data elements across trials.
- Facilitates cross-trial analysis of correlative data.
- Maintains and operates the growing central immuno-oncology database.
- Sends data to the CRDC for sharing with the general research community.
Clinical Sample Management System (CSMS)
- Tracks and manages specimens among the many network sites.
The Partnership for Accelerating Cancer Therapies (PACT)
- Provides significant financial and scientific support to the network, including additional clinical trials for collaboration.
- A public-private research collaboration among NIH, FNIH, FDA, and 12 leading pharmaceutical companies.
Learn more about the FNIH PACT.
Principal Investigators
Dana-Farber Cancer Institute
Catherine J. Wu, M.D.
Chief, Division of Stem Cell Transplantation and Cellular Therapies
Professor of Medicine, Harvard Medical School
Stephen Hodi, M.D.
Director, Melanoma Center and Center for Immuno-Oncology
Professor of Medicine, Harvard Medical School
Icahn School of Medicine at Mount Sinai
Sacha Gnjatic, Ph.D.
Associate Director, Human Immune Monitoring Center, Mount Sinai
Professor, Immunology & Immunotherapy; Medicine, Hematology and Medical Oncology; Pathology, Molecular and Cell Based Medicine; Oncological Sciences
Seunghee Kim-Schulze, Ph.D.
Facility Director, Human Immune Monitoring Center, Mount Sinai
Associate Professor, Immunology and Immunotherapy
MD Anderson Cancer Center
Ignacio Wistuba, M.D.
Chair and Professor, Department of Translational Molecular Pathology
Professor, Department of Thoracic/Head and Neck Medical Oncology
Co-Director, Division of Pathology/Lab Medicine
Faculty, University of Texas Graduate School of Biomedical Sciences
Cara Haymaker, Ph.D.
Director, Oncology Research and Immuno-Monitoring (ORION) Core
Associate Professor, Department of Translational Molecular Pathology
Gheath Alatrash, M.D., Ph.D.
Associate Professor, Department of Hematopoietic Biology and Malignancy
Stanford University
Holden Maecker, Ph.D.
Professor (Research), Microbiology and Immunology
Director, Human Immune Monitoring Center and Service Centers and Enabling Technologies
Sean Bendall, Ph.D.
Associate Professor, Pathology
Data Sharing through the NCI Cancer Research Data Commons (CRDC)
Clinical and biomarker data from CIMAC-CIDC correlative studies will be placed in the NCI Cancer Research Data Commons (CRDC) for sharing with the research community.
Those wishing to request data should submit their request to the database of Genotypes and Phenotypes (dbGaP). If the request is approved, requesters would download the data from NCI’s Data Commons Framework Services (DCFS) portal.
The attached brief guide lists the data available (as well as data being prepared for availability) and explains how to request and download data from CIMAC-CIDC studies. Please note: As described in the guide, researchers must register for an NIH eRA Commons account and apply for data access authorization to access the data.
Standardized Assays
The CIMACs offer comprehensive profiling of specimens using standardized, validated assays.
The NCI Biospecimen Research Database contains the Standard Operating Procedures and analytical performance/validation reports for the CIMAC assays.
NIH reviewers review and approve all assays for validation and analytical performance.
Assays
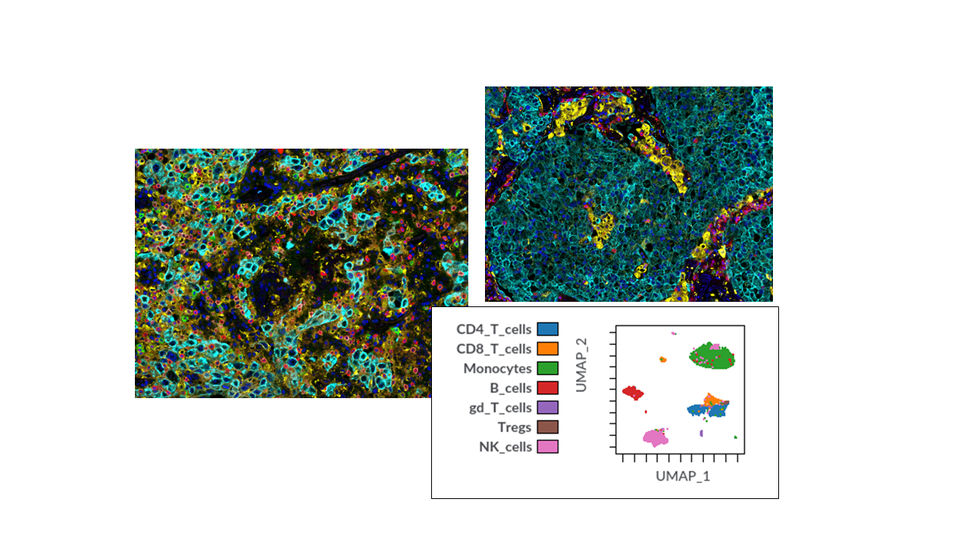
- Tissue-imaging assays
- Multiplex immunofluorescence
- Multiplex immunohistochemistry
- Singleplex immunohistochemistry (e.g., PD-L1)
- Multiplexed Ion Beam Imaging (MIBI)
- Spatial transcriptomics (Visium, GeoMx)
- Spatial tissue imaging
Soluble analyte assays
- Olink cytokine analysis
- ELISA Grand Serology
- NULISA
Immune-cell profiling assays
- Mass Cytometry (CyTOF)
- EliSPOT
Sequencing assays
- RNA sequencing
- Whole Exome Sequencing (WES)
- NanoString
- TCRseq DNA-based (via Adaptive Immunosequencing)
- TCRseq RNA-based
- ATAC-seq (epigenomics/regulomics)
- Circulating tumor DNA (ctDNA) (via The Broad Institute)
- 16S microbiome sequencing (stool)
Single-cell RNA sequencing
- Single-nucleus RNA sequencing
- Single-cell TCR sequencing
- Single-cell ATAC-Seq
- Extracellular vesicle small RNA sequencing
- CITE-seq
Assay Harmonization
The network harmonized a core set of their assays to reduce data variability among assay sites to facilitate cross-trial analysis of correlative data:
Clinical Data Standardization and Specimens
Clinical Data Collections
CIDC collects clinical data that span the following range of information for CIMACs and trial teams to analyze with the assay data:
- Demographics
- Patient history
- Disease characteristics
- Treatment
- Response
- Adverse events
CIDC uses the following template and instructions to collect the clinical data:
- Template of clinical data elements used in CIMAC-CIDC studies (Excel)
- Instructions for use of template of clinical data elements (Word)
Specimen standardization
In their “specimen collection umbrella,” CIMACs describe standardized specimen collections to support multi-assay specimen profiling:
Clinical Trial Collaborations and Publications
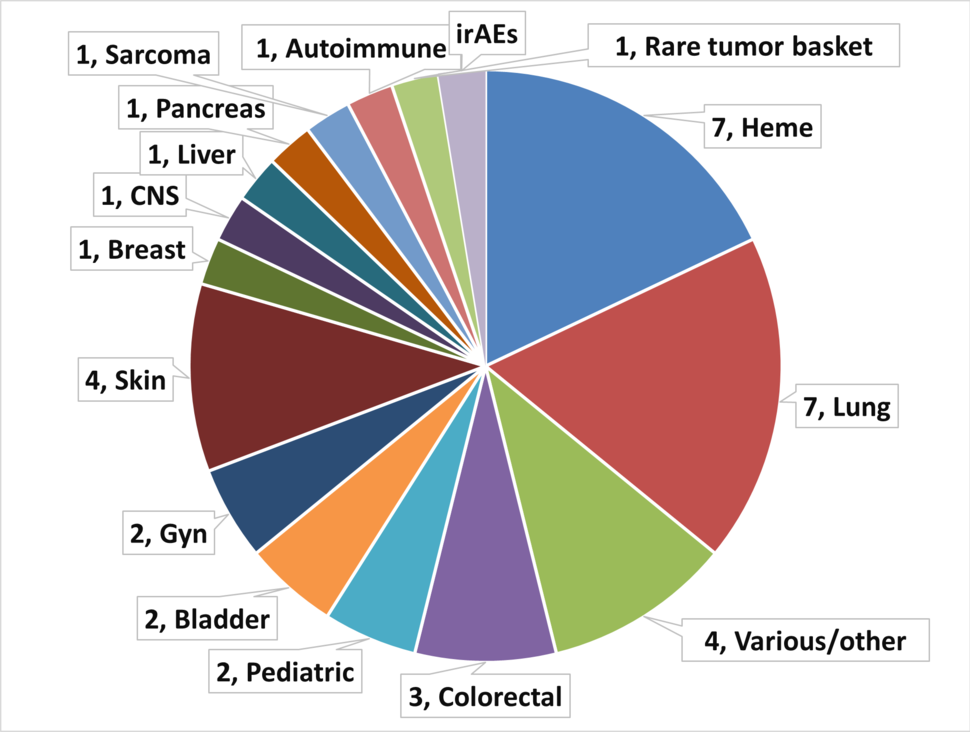
The CIMACs-CIDC Network collaborates with more than 35 clinical trials of immunotherapy in various cancer disease settings. The pie chart below depicts the variety of clinical trials by cancer type, followed by a list of trials, and biomarker publications from these trials.
Individual CIMACs and CIDC have published more than 200 additional manuscripts citing CIMAC-CIDC funding support. These publications offer insights into the mechanisms of tumor immunity and the methodologies for studying them.
The network’s Human Material Transfer Agreement (HMTA) describes the legal and regulatory terms governing collaborations with the CIMAC-CIDC Network.
Clinical trials collaborating with the CIMACs-CIDC Network and biomarker publications
| Trial ID | Description |
|---|---|
| 9204 | A Phase I/IB Study of Ipilimumab or Nivolumab in Patients with Relapsed Hematologic Malignancies After Allogeneic Hematopoietic Cell Transplantation
|
| 10013 | Randomized Phase 2 Study of Neoadjuvant Chemotherapy, Carboplatin and Paclitaxel, with or Without Atezolizumab in Triple Negative Breast Cancer (TNBC)
|
| 10021 | A Phase 2 Study of MEDI4736 (Durvalumab) and Tremelimumab Alone or in Combination with High or Low-Dose Radiation in Metastatic Colorectal and NSCLC
|
| 10026 | A Phase 1 Study of Ipilimumab in Combination with Decitabine in Relapsed or Refractory Myelodysplastic Syndrome/Acute Myeloid Leukemia
|
| 10057 | A Phase II Study of Talimogene Laherparepvec Followed by Talimogene Laherparepvec + Nivolumab in Refractory T Cell and NK Cell Lymphomas, Cutaneous Squamous Cell Carcinoma, Merkel Cell Carcinoma, and Other Rare Skin Tumors |
| 10061 | A Phase 1 Study of MK-3475 (Pembrolizumab) in Combination with Recombinant Interleukin-12 in Patients with Solid Tumors |
| 10104 | A Randomized Phase 2 Study of Cabozantinib in Combination with Nivolumab in Advanced, Recurrent Metastatic Endometrial Cancer |
| 10166 | A Phase 2 Study of Atezolizumab and Cobimetinib in PD-1/PD-L1 Inhibitor Resistant or Refractory Non-Small Cell Lung Cancer |
| 10204 | A Phase Ib Study of Nivolumab in Patients with Autoimmune Disorders and Advanced Malignancies (AIM-NIVO) |
| 10208 | A Phase I Study of Anetumab Ravtansine in Combination with Either Anti-PD-1 Antibody, or Anti-CTLA4 and Anti-PD-1 Antibodies or Anti-PD-1 Antibody and Gemcitabine in Mesothelin-Positive Advanced Pancreatic Adenocarcinoma |
| 10221 | A Phase I/II Biomarker Driven Combination Trial of Copanlisib and Immune Checkpoint Inhibitors in Patients with Advanced Solid Tumors |
| 10276 | A Phase I/II Study of M3814 and Avelumab in Combination with Hypofractionated Radiation in Patients with Advanced/Metastatic Solid Tumors and Hepatobiliary Malignancies |
| 10300 | BLockade of PD-1 Added to Standard Therapy to Target Measurable Residual Disease in Acute Myeloid Leukemia 1 (BLAST MRD AML-1): A Randomized Phase 2 Study of the Anti-PD-1 Antibody Pembrolizumab in Combination with Conventional Intensive Chemotherapy as Frontline Therapy in Patients with Acute Myeloid Leukemia |
| 10334 | BLockade of PD-1 Added to Standard Therapy to Target Measurable Residual Disease in Acute Myeloid Leukemia 2 (BLAST MRD AML-2): A Randomized Phase 2 Study of the Venetoclax, Azacitadine, and Pembrolizumab (VAP) Versus Venetoclax and Azacitadine as First Line Therapy in Older Patients with Acute Myeloid Leukemia (AML) Who Are Ineligible or Who Refuse Intensive Chemotherapy |
| 10347 | A phase I study with an expansion cohort of duvelisib and nivolumab in mycosis fungoides (MF) and Sézary syndrome (SS) |
| 14-C-0059G | A Phase I Trial of T Cells Expressing an anti-GD2 Chimeric Antigen Receptor in Children and Young Adults with GD2+ Solid Tumors
|
| 18-279 (NCT03929029) | Neoantigen Vaccine Plus Locally Administered Ipilimumab and Systemic Nivolumab in Advanced Melanoma
|
| 2017-0349 | A Phase I Study of Nivolumab in Combination with Ipilimumab for the Treatment of Patients with High Risk or Refractory/Relapsed Acute Myeloid Leukemia Following Allogeneic Stem Cell Transplantation |
| 21-066 (NCT04930783) | A Phase Ib Study of NeoVax in Combination With CDX-301 and Nivolumab and in Patients With Melanoma |
| A151804 | Establishment of a National Biorepository to Advance Studies of Immune-Related Adverse Events |
| ABTC-1603 | Phase I Study of Neoadjuvant GMCITM plus Immune Checkpoint Inhibitor Combined with Standard of Care for Newly Diagnosed High-Grade Gliomas
|
| BACCI | BACCI: A Phase II Randomized, Double-Blind, Placebo-Controlled Study of Capecitabine Bevacizumab plus Atezolizumab versus Capecitabine Bevacizumab plus Placebo in Patients with Refractory Metastatic Colorectal Cancer |
| CA027-005 | A Phase 2a Multi-Cohort Trial of Neoadjuvant Nivolumab + BMS-813160 (CCR2/5i) or BMS-986253 (anti-IL-8) for NSCLC or HCC |
| E4412 | A Phase I Study with an Expansion Cohort/Randomized Phase II Study of the Combinations of Ipilimumab, Nivolumab and Brentuximab Vedotin in Patients with Relapsed/Refractory Hodgkin Lymphoma
|
| EA6174 | A Phase III Randomized Trial Comparing Adjuvant MK-3475 (Pembrolizumab) to Standard of Care Observation in Completely Resected Merkel Cell Carcinoma |
| EAY131-Z1D | Molecular Analysis for Therapy Choice (MATCH) — MATCH Treatment Subprotocol Z1D: Phase 2 Study of Nivolumab in Patients with Mismatch Repair Deficiency
|
| GU16-257 | Neoadjuvant gemcitabine, cisplatin, plus nivolumab in patients with muscle-invasive bladder cancer with selective bladder sparing
|
| GU16-287 | Randomized phase 2 trial of gemcitabine + carboplatin + nivolumab versus gemcitabine + oxaliplatin + nivolumab in cisplatin-ineligible patients with metastatic urothelial cancer |
| LuTK02 | GMCI plus Standard of Care Immune Checkpoint Inhibitor for Stage III/IV NSCLC Patients |
| NRG-GI002 | A Phase II Clinical Trial Platform of Sensitization Utilizing Total Neoadjuvant Therapy (TNT) in Rectal Cancer |
| NRG-GY021 | A Phase II Randomized Trial of Olaparib Versus Olaparib Plus Tremelimumab in Platinum-Sensitive Recurrent Ovarian Cancer |
| NRG-LU004 | Phase I Trial of Accelerated or Conventionally Fractionated Radiotherapy Combined with MEDI4736 (Durvalumab) in PD-L1 High Locally Advanced Non-Small Cell Lung Cancer (NSCLC) (ARCHON-1) |
| OPTIMAL (TOP-1705) | A Phase II Clinical Trial of Combination Nivolumab (Opdivo), Ipilimumab (Yervoy), and Paclitaxel in Patients With Untreated Metastatic Non-Small Cell Lung Cancer (NSCLC) (The OPTIMAL Trial) [TOP 1705] |
| PED-CITN-02 | GD2-CAR PERSIST: Production and Engineering of GD2-Targeted, Receptor Modified T Cells (GD2CART) for Osteosarcoma or Neuroblastoma to Increase Systemic Tumor Exposure |
| S1609 | DART: Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors
|
| S1400I | A Phase III Randomized Study of Nivolumab Plus Ipilimumab Versus Nivolumab for Previously Treated Patients with Stage IV Squamous Cell Lung Cancer and No Matching Biomarker (Lung-Map Sub-Study)
|