The Clinical Proteomic Tumor Analysis Consortium (CPTAC) is a nationwide collaborative effort to accelerate the understanding of the molecular basis of cancer through the application of large-scale proteome and genome analysis, or proteogenomics.
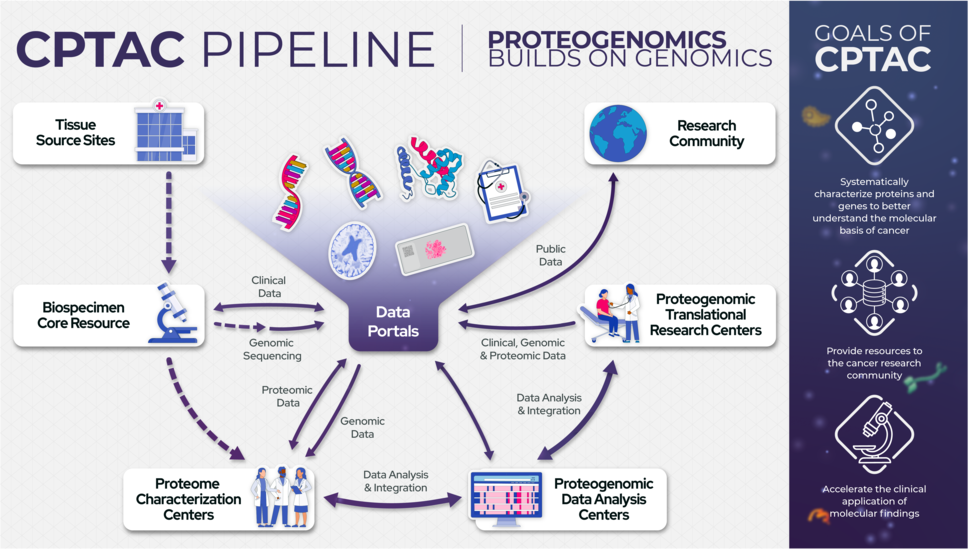
Launched in 2011, CPTAC implements this collaborative effort through a standardized workflow that brings together biospecimen collection and processing resources, genome and proteome characterization centers, data coordinating and data analysis centers and translational research centers.
The overall objective of CPTAC is to systematically identify proteins and related biological processes that derive from alterations in cancer genomes. This is done in order to add a complementary functional layer of protein biology to genomic profiles. This “proteogenomic” approach enhances our understanding of cancer by helping prioritize cancer driver genes and improving patient subtyping. This approach also identifes dynamic alterations in posttranslational modifications responsible for the dysregulation of cancer signaling networks and pathways and elucidates the molecular basis of drug response and resistance to therapies.
All CPTAC investigators collaborate, share data and expertise across the consortium, and participate in consortium activities. Data (genomics, proteomics, imaging), assays, reagents, and open-source analytical tools are made available to the public as a Community Resources to accelerate cancer research and advance patient care.
Research activities are coordinated by multidisciplinary and collaborative teams of scientists from institutions nationwide that comprehensively characterize tumors, perform integrative proteogenomic analyses, and apply proteogenomics methods to elucidate drug response and resistance in clinical trials. These teams comprise:
- Proteome Characterization Centers (PCCs): PCCs work as a group to comprehensively characterize NCI-provided treatment-naïve biospecimens (genomically characterized) using high-throughput, analytically validated, standardized, state-of-the-art proteomic technologies/workflows, to ensure the reliability and usefulness of the overall data collection effort.
- Proteogenomic Translational Research Centers (PTRCs): PTRCs address questions of biology in the context of the NCI-sponsored clinical trial, using an integration of proteomics and genomics, focusing on drug response and resistance to therapies.
- Proteogenomic Data Analysis Centers (PGDACs): PGDACs work as a group to integrate, visualize, and analyze CPTAC data across different data types (genomics, transcriptomics, and proteomics). They aim to identify potentially cancer-related molecular alterations at a linear sequence and network and pathway level. They also elucidate how distinct changes at the protein level are related to abnormalities in cancer genomes.
Rigor and Reproducibility
- Commitment To Standards: CPTAC is committed to applying the highest analytical and computational standards, while providing resources and reagents to the public that enable cancer researchers to effectively and reproducibly use proteomic and proteogenomic approaches.
- Commitment To Quality: CPTAC is committed to using the latest state-of-the-art technology to provide timely, accurate and reliable omic data, as well as other types of data in cancer biology and related technologies.