Office of the Associate Director
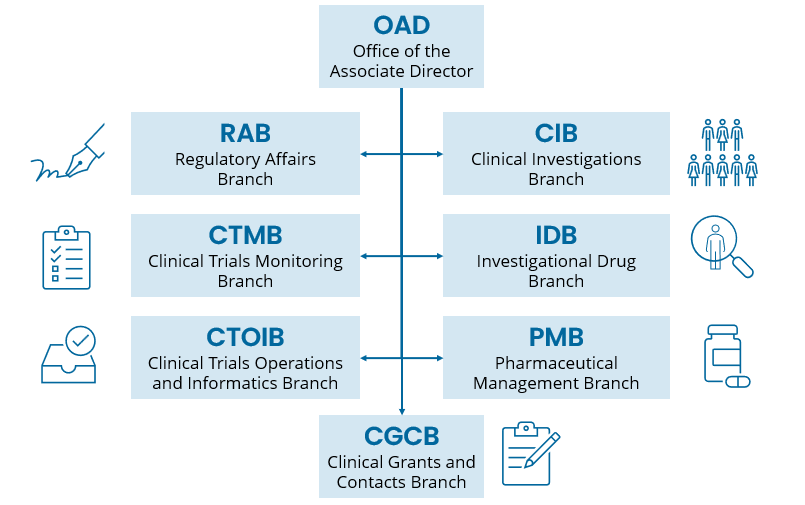
The CTEP Office of the Associate Director (OAD) provides programmatic and management support to activities across CTEP's seven branches.
Clinical Oversight of CTEP-Supported Network Trials
Two branches consist primarily of physicians providing clinical oversight and direction to the development of agents under CTEP IND. They also oversee the design and conduct of early and late-phase clinical trials of novel cancer treatments:
- The Investigational Drug Branch (IDB) is responsible for the clinical development of anticancer agents that are being developed under CTEP IND. IDB has primary oversight of the Experimental Therapeutics Clinical Trials Network (ETCTN).
- The Clinical Investigations Branch (CIB) is responsible for the scientific coordination and oversight of definitive, practice-changing clinical trials of innovative oncology treatments. CIB has primary oversight of the National Clinical Trials Network (NCTN).
Network Clinical Trial Infrastructure and Support
Four branches provide and oversee critical services for trial activation and operations across CTEP-supported networks:
- The Clinical Trials Monitoring Branch (CTMB) provides quality assurance, including auditing and monitoring, for CTEP-supported clinical trials networks to ensure patient safety and data integrity.
- The Clinical Trials Operations and Informatics Branch (CTOIB) improves clinical trial development and conduct by providing efficient business practices, informatics tools, and central review processes for NCI-supported clinical trials networks.
- The Regulatory Affairs Branch (RAB) consists of two groups. The Agreement Coordination Group (ACG) negotiates and reviews agreements with industry collaborators for investigational drug development. The Drug Regulatory Group (DRG) is responsible for regulatory filings and meetings for trials conducted under CTEP IND and ensuring compliance with regulatory requirements.
- The Pharmaceutical Management Branch (PMB) provides pharmaceutical services ensuring appropriate access to CTEP's investigational agents, including online agent management support and site and personnel registration.
Investigator-Initiated Clinical Research
CTEP's Clinical Grants and Contracts Branch (CGCB) oversees a broad portfolio of CTEP-supported grants and contracts.