NExT Research and Development Activities
The NExT Pipeline is divided into 4 discovery stages (Target Validation, Exploratory Screen Development, Screening/Hit-to-Lead, and Lead Development) and 2 early development stages (Candidate Selection/Nomination and Preclinical Evaluation (including all pre-Investigational New Drug (IND) activities and regulatory support). Clinical evaluation of compounds is managed and developed through collaborations with the Cancer Therapy Evaluation Program (CTEP) Investigational Drug Branch (IDB) which support activities for clinical trials from early phase first in human studies through Phase II combination studies.
Examples of Activities that Support NExT Discovery and Development Stages
Discovery Stages
Target Validation
- Evaluate and define the role of therapeutic target in disease initiation and/or progression
- Determine if there is evidence that demonstrates the target is associated with disease pathology
- Identify causal/driver variant(s)/patient populations with genetic backgrounds susceptible to intervention
Exploratory Screen Development
- Conduct a technology overview
- Develop a screening strategy
- Identify potential biomarkers (efficacy/surrogate)
- Develop a strategy for “clinical readiness”
- Prepare medical needs assessment
- Prepare project operational plan
Screening/Hit-to-Lead
- Run high-throughput screening
- Assess mechanism of action for link to disease
- Assess mechanism of action for link to disease
- Determine desirable potency
- Determine evidence of structure–activity relationship
- Evaluate functional activity in vitro
- Determine selectivity for target
- Evaluate physicochemistry (Rule-of-Five compliance)/in silico modeling
- Evaluate PK and PD using best available tools
- Assess amenability to synthesis
- Evaluate stability
Lead Development
- Establish laboratory objectives for clinical efficacy
- Resolve IP issues
- Evaluate activity in validated disease models
- Evaluate physicochemistry
- Differentiate leads from competitors and current therapies
- Evaluate preliminary safety issues
- Develop PD and toxicology biomarker assays(s)
- Assess achievability of human PK/PD profile
- Assess feasibility of scale-up and bulk synthesis
Early Development Stages
Candidate Selection/Nomination
- Evaluate synthesis and proposed clinical formulation
- Evaluate biopharmaceutical properties (absorption in rodents and non-rodents, clearance, and bioavailability)
- Assess potency against clinical efficacy
- Evaluate biodistribution
- Evaluate clinical readiness of PK/PD assay(s) and specimen handling SOPs
- Assess amenability to imaging
- Evaluate safety issues (most sensitive species) in range-finding toxicology studies
- Prepare clinical development plan
Preclinical Evaluation
- Manufacture GMP-grade bulk drug/active pharmaceutical ingredient (API)
- Conduct IND-directed toxicology studies including toxicokinetics
- Determine preclinical MTD, DLTs, and starting dose
- Validate PK/PD assay(s) and specimen handling SOPs
- Develop and validate product characterization and release assays
- Characterize clinical product
- Prepare CMC package and toxicology summary report
- Prepare and review clinical protocol at each participating site
- Prepare and file IND
Patent Rights and Licensing Agreement Terms
Applicants will retain ownership of any intellectual property that they bring to the program. They may be offered licensing rights on inventions generated by the government or its agents in furtherance of the program, depending on the stage of development.
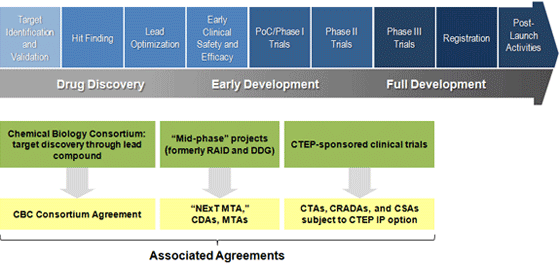
NExT Pipeline Agreement Types by Project Phase
Chemical Biology Consortium (CBC) Participants Agreement - Discovery Stages
Work conducted by Master Service Agreement (MSA) holders and other signatories of the CBC Participants Agreement is subject to the Bayh-Dole Act (35 U.S.C. § 200, et. seq.). Additionally, all CBC members are expected to follow the principles outlined in the CBC Participants Agreement
NExT Material Transfer Agreement (MTA) - Early Development/Pre-clinical Stages
Applicants whose projects enter the pipeline after the lead development stage but prior to clinical evaluation (including activities formerly under the RAID Program) are expected to execute a NExT MTA. The terms of this agreement will govern the collaboration between the NCI and the applicant on early development stage preclinical projects.
Clinical Trial, Collaborative Research and Development, and Clinical Supply Agreements - CTEP Sponsored Clinical Trials
Currently, the language for the management of patent rights under agreement with NCI for CTEP-sponsored clinical trials states that clinical trial institutions will grant the NExT Collaborator a paid-up non-exclusive research use license and offer the time-limited first option to negotiate a royalty-bearing license on any clinical trial institution inventions generated from the NExT Collaboration. For combination studies, all collaborators providing investigational agents for combination studies will receive a non-exclusive, royalty-free commercialization license to any inventions generated under the course of study that incorporate their agent. These agreements include the Clinical Trials Agreement (CTA), Cooperative Research and Development Agreement (CRADA), and Clinical Supply Agreement (CSA).
Intellectual Property
NCI Employee Inventions
The rights that NCI retains in employee inventions depend on the stage of development and the agreement negotiated between the NCI and the parties.
For early stage CBC agreements, most of the work will be conducted by contract and non-contract CBC participants as opposed to Federal laboratories. As such, there will be few if any government inventions generated under these studies. Should conception or reduction to practice occur within the Federal government, the NCI will follow the licensing guidelines established by the NIH Office of Technology Transfer.
For mid stage NExT MTA projects, normally the NCI will not acquire intellectual property rights to inventions made by its employees using NExT Collaborator Materials under NExT unless the NExT Collaborator and the NCI mutually agree that to do so would be in the best interest of the NExT Collaborator and the invention's further development. The NCI will inform the NExT Collaborator of any such inventions, and, after consultation with the NExT Collaborator, the NCI will decide whether or not to file a patent application on any such invention. If the NCI does file a patent application, the NExT Collaborator will be given an opportunity to negotiate for a license in accordance with the procedures set forth in 37 CFR Part 404.
For late stage CTEP projects, the Collaborator will have licensing rights in any NCI employee subject invention as defined by the CRADA agreement between the NCI and the Collaborator. The licensing terms will be described in the model CRADA and will be substantially similar to the language found in the CTEP IP Option.
Contract Research Organizations (CRO)
The NCI will inform the NExT Collaborator of the results of the NCI contractor’s or subcontractor’s research using the NExT Collaborator’s Materials.
Inventions made by the CROs are subject to the Bayh-Dole Act; however, to encourage applicants to participate in the NExT Program, such NCI contractors and subcontractors have agreed to include, as a term and condition of their funding agreements, an agreement to offer to the NExT Collaborator a first option to negotiate a license to subject contractor or subcontractor inventions made using the NExT Collaborator’s Research Material.
Data Access and Sharing
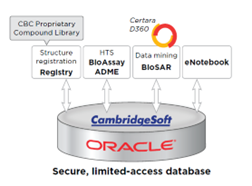
Data generated by NExT services are stored in highly secure, password-protected databases and file storage systems operating inside the NIH firewall. A user must hold a NIH account and obtain appropriate approval to gain access to the databases. NIH accounts are only granted to those who have completed an NIH security awareness training course and account access is enforced with strict password criteria. Database access is granted on a “need-to-know” basis, accompanied by a further restraint that data are not to be shared with any individuals without direct access.