MDNet Supports MyeloMATCH
NCI's Molecular Diagnostic Network (MDNet) is an NCI-funded network of laboratories that will perform all the molecular and cellular biomarker assays for NCI's three new precision medicine trials for the purpose of assigning patients to treatment.
One of the trials for which MDNet will perform molecular testing is MyeloMATCH, a novel precision medicine umbrella trial evaluating treatments for acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS). Previously untreated people with AML and MDS will be assigned based on their molecular and clinical features to a series of randomized clinical trials. The clinical investigations will target progressively smaller residual tumor burden more precisely over time.
NCI-MATCH, ComboMATCH and the Designated Laboratory Network
The NCI-MATCH (Molecular Analysis for Therapy Choice) trial, supported by the NCI-sponsored National Clinical Trials Network (NCTN), was led by CDP and the ECOG-ACRIN Cancer Research Group with assistance from DCTD’s Cancer Therapy Evaluation Program (CTEP), Biometrics Research Program (BRP), and additional committees.
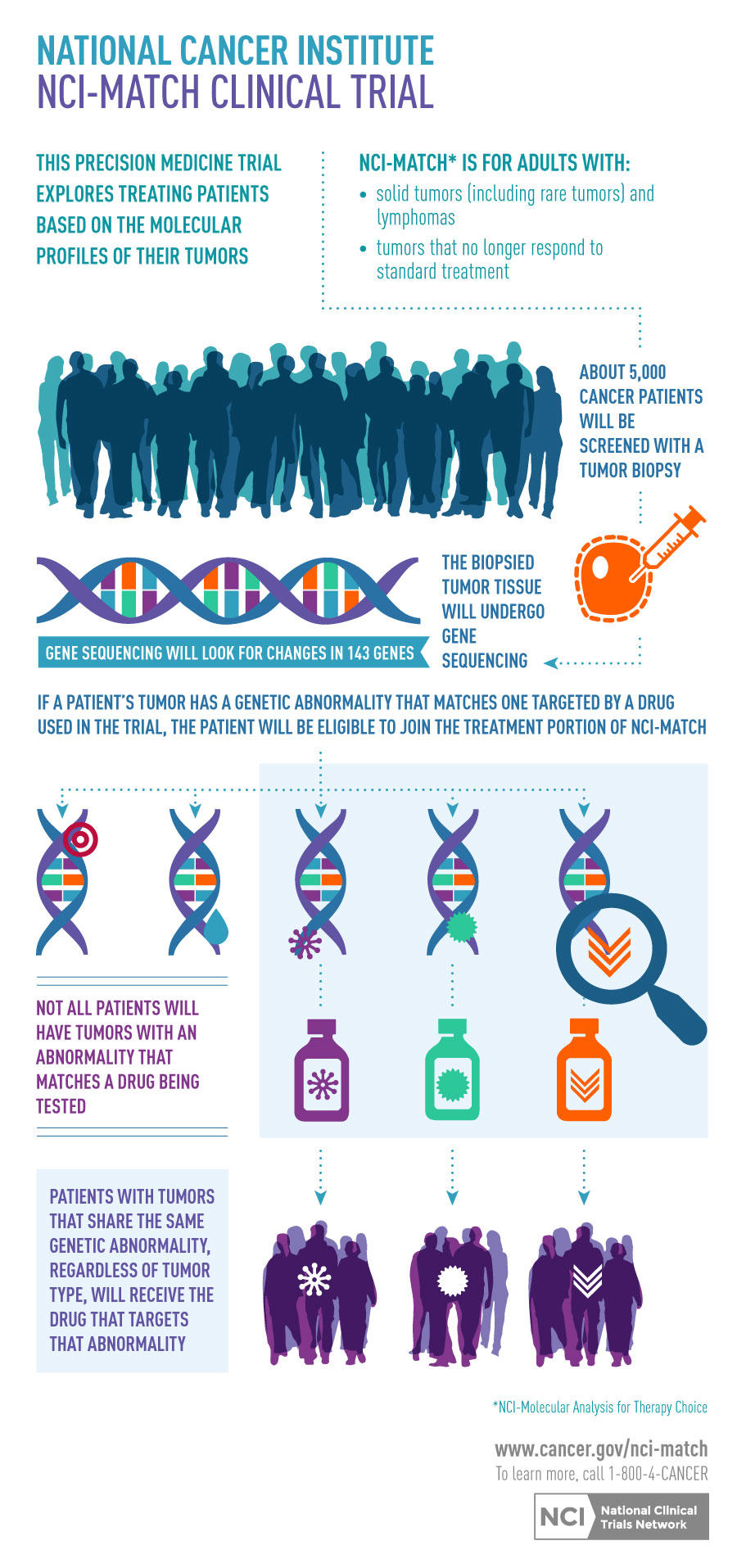
NCI-MATCH sought to determine the effectiveness of treatment based on matching a molecular targeted agent to a specific genetic abnormality of a tumor, regardless of tumor type. The trial showed that people with advanced cancer may benefit from genomic sequencing to help plan their treatment. The study assigned patients with solid tumors, myeloma, or lymphoma, who had progressed on standard therapy, to receive treatment based on genetic changes found in their tumors that were found using genomic sequencing and other tests performed at NCI-MATCH Designated Laboratories. 1,201 people were enrolled in the trial on 38 different arms. The trial is no longer enrolling new patients, but follow-up will continue for everyone who took part in the trial for up to 3 years after treatment. Visit the ECOG-ACRIN site to see a list of NCI-MATCH findings and publications.
New precision medicine trials ComboMATCH, MyeloMATCH, and ImmunoMatch (iMATCH) have succeeded the landmark NCI-MATCH trial and continue to build on the trial results.
Originating with NCI-MATCH, the Designated Laboratory Network consists of commercial and academic laboratories that have been evaluated with quality metrics for concordance with the study central assay, and all operate under the Clinical Laboratory Improvement Amendments (CLIA) standards. The labs perform testing to molecularly profile patients’ tumors in the clinical setting.
The Designated Laboratory Network is currently supporting ComboMATCH, a group of trials testing targeted drug combinations in people with cancer. The trial's goal is to overcome drug resistance to single-agent therapy through the development of targeted agent combinations based on the gene signatures of specific cancers. NCI and the NCTN support ComboMATCH, with ECOG-ACRIN coordinating. People are referred to trials in ComboMATCH from the External Designated Laboratory Network if routine NGS of their tumor reveals at least one qualifying alteration.
NCI-COG Pediatric MATCH
NCI-COG Pediatric MATCH was a phase 2 clinical trial developed and led jointly by NCI and the Children’s Oncology Group (COG), enrolling children and adolescents with advanced solid tumors including non-Hodgkin lymphomas, brain tumors, and histiocytosis, who had progression on standard therapy or recurrence after treatment. Participants were assigned to treatment arms based on the detection of certain genetic abnormalities using tumor DNA sequencing. As of January 2025, enrollment in Pediatric MATCH has completed; follow-up will continue for all patients receiving treatment for a total of 5 years from enrollment.