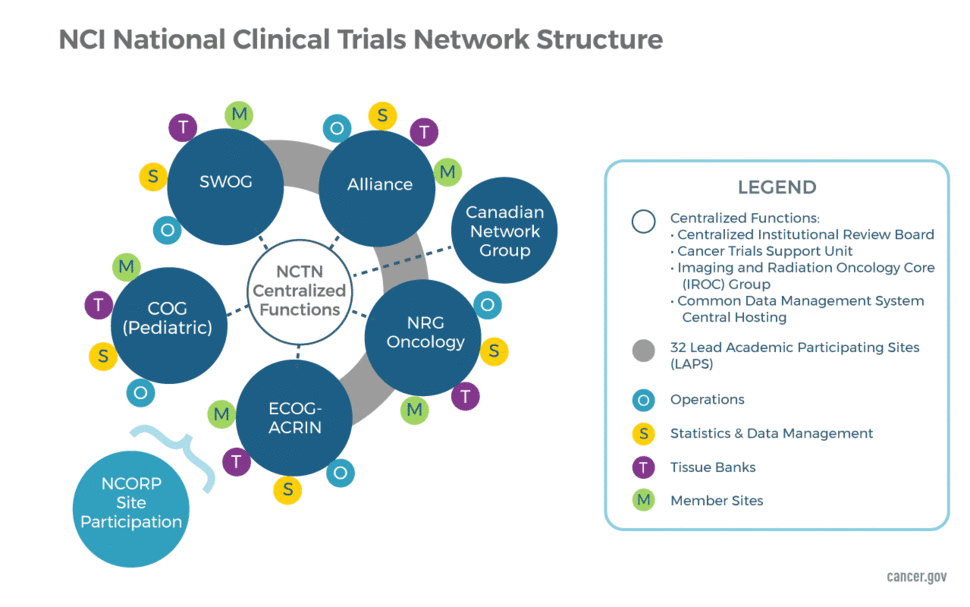
NCTN Network Groups
The NCTN includes five U.S. Network Groups (one pediatric and four adult focused Groups) and one Canadian Collaborating Network Group. Each U.S. Network Group is supported by multiple cooperative agreements: one grant to the NCTN Group Operations Center, one grant to the Group Statistics and Data Management Center (SDMC), and one grant to the Group Biobank. The Canadian Collaborating Network Group is supported by a single cooperative agreement and is responsible for partnering with the U.S. Network Groups in the conduct of large-scale, multi-site clinical trials. It also helps to provide regulatory expertise/oversight for the conduct of NCTN trials in Canada.
The NCTN Group Operations Centers provide scientific leadership for developing and implementing multi-disciplinary, multi-institutional trials in a range of diseases and special populations with specific scientific strategy and goals. The Operations Centers are responsible for developing new protocols and managing the regulatory, financial, membership, and scientific committees of each group. It is also responsible for the conduct of the studies the group leads, including safety monitoring. The Operations Centers are expected to be closely integrated with their corresponding Statistics and Data Management Center in all aspects of trial operations through jointly developed policies and procedures for clinical trial development and conduct.
The NCTN Group Statistical and Data Management Centers are responsible for providing the statistical expertise required to ensure effective scientific design and conduct of clinical trials. They also provide leadership in innovation of statistical methodology. These centers are also responsible for data management, data analysis, and statistical analysis for NCTN trials led by their affiliated Network Group Operations Center, as well as for translational and other ancillary studies associated with the trials.
NCTN clinical trials are uniquely positioned to provide high-quality biologic specimens associated with detailed treatment histories, recurrence data, and careful follow-up from patients over long periods of time. The NCTN Biobanks constitute a large collection of well-annotated biospecimens from people enrolled on NCTN clinical trials run through the NCTN Groups. Although funding for sites to collect biospecimens is provided under the NCTN Group Operations Center grants along with scientific review for use of biospecimens, funding for the NCTN Group Biobanks is provided by a separate grant program under the oversight of the NCI Cancer Diagnosis Program.
The five U.S. NCTN Groups and the Canadian Collaborating Network are:
- Alliance for Clinical Trials in Oncology
- ECOG-ACRIN Cancer Research Group
- NRG Oncology
- SWOG
- Children’s Oncology Group (COG)
- Canadian Cancer Trials Group (CCTG)
Clinical trials led by NCTN Groups may utilize the IROC, ITSAs, and the tissue banks, when appropriate, to support the scientific needs of the trial.
Lead Academic Participating Sites (LAPS)
As part of the 2019 recompetition, thirty-two U.S. academic research institutions were selected as LAPS funded through UG1 cooperative agreements. These sites are academic research institutions that have fellowship training programs that have demonstrated their ability to enroll high numbers of patients onto NCTN trials. They also provide scientific leadership in trial design and conduct. The LAPS grant component of the NCTN provides additional support to the selected institutions for the increased level of patient data management work required because of their high enrollment rate.
The current 32 LAPS grantees are:
- Case Western Reserve University – Case Comprehensive Cancer Center
- Dana Farber/Harvard Cancer Center
- Duke Cancer Institute at Duke University Medical Center
- Emory University – Winship Cancer Institute
- Fred Hutchinson Cancer Research Center
- Johns Hopkins University - Sidney Kimmel Comprehensive Cancer Center
- Mayo Clinic Cancer Center
- Medical College of Wisconsin
- Memorial Sloan Kettering Cancer Center Norris Cotton Cancer Center at Dartmouth Hitchcock Medical Center
- Northwestern University – Robert H. Lurie Comprehensive Cancer Center
- Ohio State University Comprehensive Cancer Center
- Roswell Park Cancer Institute
- Sidney Kimmel Cancer Center at Jefferson Health
- University of Alabama at Birmingham
- University of California Davis Comprehensive Cancer Center
- University of Chicago Comprehensive Cancer Center
- University of Colorado Cancer Center
- University of Michigan Rogel Cancer Center
- University of North Carolina Lineberger Comprehensive Cancer Center
- University of Oklahoma – Stephenson Cancer Center
- University of Pittsburgh Cancer Institute
- University of Rochester Wilmot Cancer Institute
- University of Southern California – Norris Comprehensive Cancer Center
- University of Texas MD Anderson Cancer Center
- University of Texas Southwestern Medical Center – Harold C. Simmons Cancer Center
- University of Utah - Huntsman Cancer Institute
- University of Wisconsin Carbone Cancer Center
- Vanderbilt University Medical Center - Vanderbilt Ingram Cancer Center
- Washington University at St. Louis - Siteman Cancer Center
- Wayne State University Barbara Ann Karmanos Cancer Institute
- Yale University – Yale Cancer Center
Integrated Translational Science Awards (ITSAs)
The NCTN contains a translational component comprising five academic institutions that are funded through ITSA grants. It supports teams of translational scientists. These teams use innovative genetic, proteomic, and imaging technologies to help identify and qualify potential predictive biomarkers of response to therapy. NCTN Groups can then incorporate these biomarkers into future clinical trials. These awards leverage ongoing work in the investigators’ laboratories, which is often supported in part by other NCI grants, to assist the NCTN Groups to bring new laboratory discoveries into clinical trials. These laboratories employ cutting-edge technologies that characterize tumors and identify changes in tumor biology in response to treatment that may help explain mechanisms of treatment resistance. The five current ITSA-funded projects are:
- COG NCTN Integrated Translational Science Center for Hematopoietic Malignancies in Children; Children’s Hospital of Philadelphia
- ECOG-ACRIN Thoracic Malignancies Integrated Translational Science Center; Emory University – Winship Cancer Institute
- ECOG-ACRIN Integrated Leukemia Translational Science Center; Memorial Sloan Kettering Cancer Center
- Integrated Translational Science Center for Leukemia for Alliance & SWOG; Ohio State University Comprehensive Cancer Center
- UNC / UT National Clinical Trials Network Group Integrated Translational Science Production and Consultation Center for Alliance & NRG; University of North Carolina Lineberger Comprehensive Cancer Center
Imaging and Radiation Oncology Core (IROC)
To help monitor and ensure quality in trials that involve new imaging modalities and/or radiation therapy, the NCTN established a consolidated IROC to assist NCTN Groups using these modalities in their trials. The consolidation of these activities under the leadership of a centralized core team improves efficiency and optimizes the use of these resources by the entire network. This unique Quality Assurance (QA) entity brings together imaging QA leaders and specialists into a single, coordinated program designed to support the NCTN and other NCI-sponsored groups and networks to carry out rigorous oncologic multi-center clinical trials. Within the context of NCI-sponsored trials, the IROC is tasked with providing:
- Scientific expertise in advanced medical imaging, radiotherapy, and information technology to support establishment of appropriate QA procedures
- Consultation to the NCTN Groups in the development of research protocols early in the process to assist with hypothesis generation and trial design that can be supported by effective QA programs
- Resources for the efficient collection, qualification, analysis, archive and transfer of images, radiotherapy plans and associated clinical data
- Qualification and credentialing policies to help ensure the delivery of appropriate protocol-specified radiotherapy and advanced imaging
NCTN Steering Committees
The NCI Coordinating Center for Clinical Trials (CCCT) convenes scientific steering committees, which are composed of leading cancer experts, community oncologists, biostatisticians, translational scientists, and patient advocates, as well as NCI senior investigators. The NCTN Steering Committees are committed to increasing the exchange of information at an early stage of clinical trial development and the efficiency of clinical trial collaboration. NCTN Steering Committee activities include:
- Evaluating and prioritizing clinical trial concepts to be conducted through the NCTN at monthly meetings; sharing information at an early stage of trial development
- Reviewing and updating strategic priorities for a given disease or research area
- Assessing the strength of the clinical trial portfolio and its alignment with strategic priorities
- Convening clinical trial planning meetings to plan trials and address other trial-related issues as needed
- Forming task forces or working groups when needed to focus on a specific disease or issue