NCLN-Supported Pharmacodynamic Biomarker Assays
The National Clinical Laboratory Network (NCLN) offers validated, harmonized SOPs while implementing uniform assay workflow, instrumentation, and data analysis across network laboratories. Approved NCLN assays will be performed at the NCI’s expense, with no need to identify a funding source.
Luminex Immunoassays
AKT Multiplex Immunoassay (Panels 1-3) and ERK/MEK Multiplex Immunoassay (Panels 4-5)
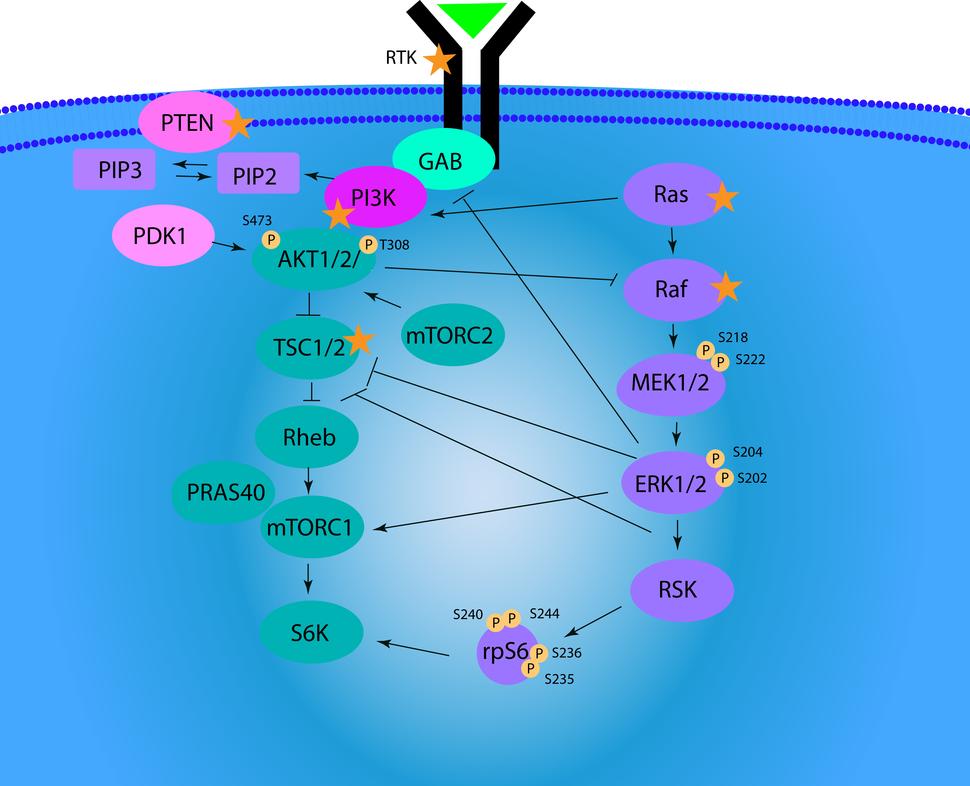
The Signaling Multiplex Immunoassays are novel isoform specific signaling multiplex immunoassay panels of 12 biomarkers for AKT and rpS6 and 8 biomarkers for MEK and ERK to measure on target or downstream pathway modulation by multiple classes of drugs including PI3K K/AKT/ mTORC and RAS/RAF/MEK/ERK inhibitors. The AKT/rpS6 multiplex immunoassays were built on the Luminex platform by grouping 12 biomarkers into three panels.
- Signaling Multiplex Panel 1 ("Total AKT/rpS6 Panel") detects total levels of AKT1, AKT2, AKT3, and rpS6. Signaling Multiplex Panel 2 ("Phospho AKT/rpS6 A Panel") detects pSer473-AKT1, pSer474-AKT2, pSer472-AKT3, and pSer235-rpS6 and Signaling Multiplex Panel 3 ("Phospho AKT/rpS6 B Panel") detects pThr308-AKT1, pThr309-AKT2, pThr305-AKT3, and pSer240/244-rpS6.
- The MEK/ERK multiplex immunoassays were built on the Luminex platform by grouping 8 biomarkers into two panels. Signaling Multiplex Panel 4 ("Total MEK/ERK Panel") measures total levels of ERK1, ERK2, MEK1, and MEK2. Signaling Multiplex Panel 5 ("Phospho MEK/ERK Panel") measures pThr202/Y204-ERK1, pThr185/Y187-ERK2, pSer218/222-MEK1, and pSer222/226-MEK2.
Assay SOPs
Specimen Collection:
- Sample Preparation
Kit Use and Data Reporting SOP:
NCI Publications
- Isoform- and Phosphorylation-specific Multiplexed Quantitative Pharmacodynamics of Drugs Targeting PI3K and MAPK Signaling in Xenograft Models and Clinical Biopsies.
- Selumetinib in adults with NF1 and inoperable plexiform neurofibroma: a phase 2 trial.
Apoptosis Multiplex Immunoassay
The Apoptosis Multiplex Immunoassay is a novel multiplex immunoassay panel of 13 biomarkers indicative of the induction, onset, and commitment to apoptosis. The multiplex immunoassays were built on the Luminex platform by grouping 13 biomarkers into three panels.
Panel 1 contains BAK, BAX, Lamin B (intact and 45 kDa fragment), and SMAC; Panel 2 contains BAD, BAX:BCL2 heterodimer, BCL-XL, BIM, and MCL1; and Panel 3 contains active caspase 3, BCL-XL:BAK heterodimer, MCL1:BAK heterodimer, and Survivin.
Assay SOPs
Specimen Collection
- Sample Preparation
- Kit Use and Data Reporting SOP
NCI Publications
- The National Cancer Institute ALMANAC: A Comprehensive Screening Resource for the Detection of Anticancer Drug Pairs with Enhanced Therapeutic Activity.
- Effect of a Smac Mimetic (TL32711, Birinapant) on the Apoptotic Program and Apoptosis Biomarkers Examined with Validated Immunoassays Fit for Clinical Use.
Multiplex Immunofluorescence Assays
ɣH2AX, pNBS1 and pKAP1 IFA with βCATN segmentation
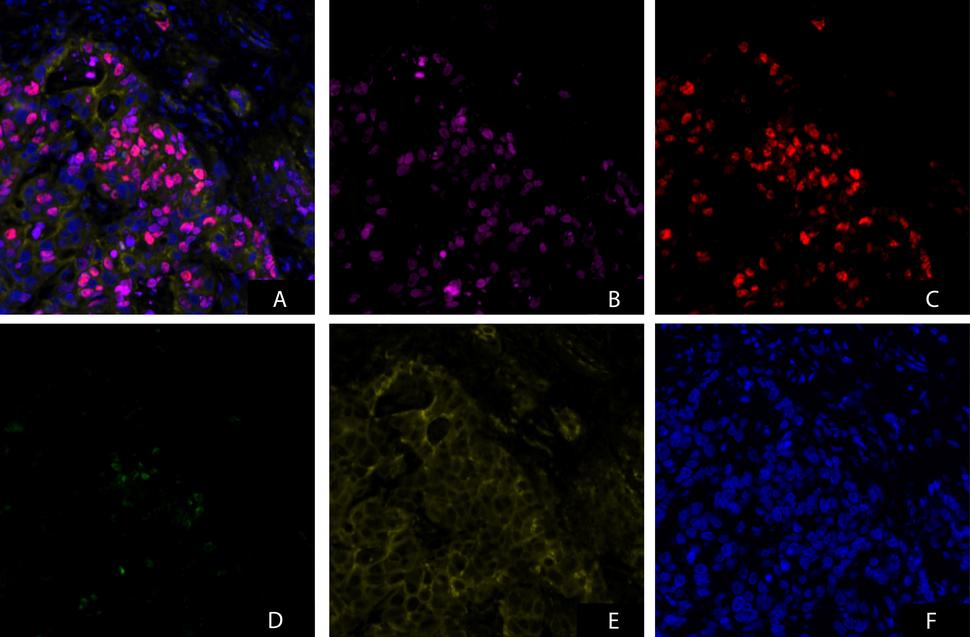
The γH2AX, pNBS1, pKAP1 IFA with βCATN segmentation is an immunofluorescence-based assay developed to quantify γH2AX, pNBS1 and pKAP1 using β-Catenin staining to segment tumor from non-tumor to precisely limit quantitation of these DNA damage response markers to the tumor cells within a tumor biopsy section.
Assay SOPs
Specimen Collection:
- Sample Preparation
- Assay SOP
Image Capture:
- Image and Data Analysis
NCI Publications
- Evaluation of Pharmacodynamic Responses to Cancer Therapeutic Agents Using DNA Damage Markers.
- The National Cancer Institute ALMANAC: A Comprehensive Screening Resource for the Detection of Anticancer Drug Pairs with Enhanced Therapeutic Activity.
- Pharmacodynamic effects of the PARP inhibitor talazoparib (MDV3800, BMN 673) in patients with BRCA-mutated advanced solid tumors.
- Phase I Study of Single-Agent AZD1775 (MK-1775), a Wee1 Kinase Inhibitor, in Patients with Refractory Solid Tumors.
Contact
PDinfo@mail.nih.gov (questions, training requests, or comments)