About the NCLN
Working with the NCLN provides multiple advantages in early-phase trials:
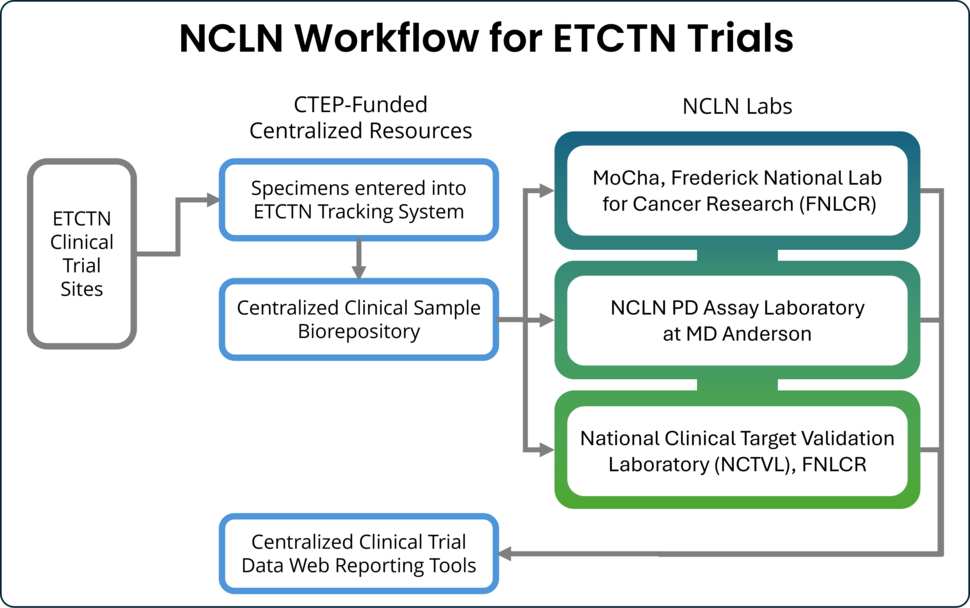
- Centralized specimen tracking and biobanking
- Uniform specimen processing and assay workflows
- Validated assays following harmonized SOPs
- Centralized web tools for data reporting
- Full funding for all CTEP-approved NCLN assays
Pharmacodynamic Biomarker Assays Available Through the NCLN
For detailed information on PD assays, visit the NCLN Pharmacodynamic Assays Page.
Multiplex Immunofluorescence Assays:
- ɣH2AX, pNBS1 and pKAP1 IFA with βCATN segmentation: quantification of 3 biomarkers that are indicative of DNA damage response
- pHH3, pY15-Cdk IFA with βCATN segmentation: quantification of 2 biomarkers to measure cell cycle control status
Luminex Immunoassays
- Apoptosis Multiplex Immunoassay, Luminex: quantification of 13 biomarkers indicative of the induction, onset, and commitment to apoptosis
- AKT Multiplex Immunoassay Panels 1-3: quantification of 12 biomarkers for AKT and rpS6 to measure on target or downstream pathway modulation
ERK/MEK Multiplex Immunoassay Panels 4-5: quantification of 8 biomarkers for MEK and ERK to measure on target or downstream pathway modulation
Investigators may prioritize panels or select a subset of available Luminex panels.
Genomics Biomarker Assays Available Through the NCLN
For detailed information on sequencing assays, visit the NCLN Genomic Assays Page.
Whole Exome Sequencing (WES): to examine genomic status across the exome, including SNVs, indels, copy number variation, tumor mutational burden (TMB), microsatellite instability (MSI), mutational signature, homologous recombination deficiency (HRD), and mismatch repair (MMR) deficiency
RNAseq: to examine gene expression, signaling pathways and gene fusion status across the transcriptome
Oncomine Panel: targeted examination of genomic status across a panel of 161 cancer-related genes
Circulating Tumor DNA (ctDNA): targeted examination of genomic status in circulating tumor DNA across 523 genes, including mutations, SNVs, translocations, copy number alterations, and indels
The NCLN Process for ETCTN Investigators
Investigators may request an NCLN assay by including it in the Biomarker Plan Table when submitting a Letter of Intent (LOI). CTEP may also recommend assays during the LOI review process.
To request an NCLN assay, download the NCLN Assay Menu (formatted for compatibility with the LOI/Protocol Biomarker Table) and copy-paste the relevant table rows into the Biomarker Table in your LOI.
Overview of the NCLN Review Process:
- LOI submitted with NCLN assay request
- LOI provisional approval
- NCLN review meeting (based on the LOI; no additional materials needed from study team)
- NCLN consensus review issued to study team (if applicable)
- NCLN call (if needed or requested to answer any questions)
- NCLN response submitted by study team (if applicable)
- NCLN decision letter issued
Once the NCLN decision letter is received and the LOI receives full approval, NCLN assays can be incorporated into the developing protocol document. CTEP provides standardized protocol language for NCLN specimen processing and shipping instructions. Specimens designated for NCLN-based biomarkers must be entered into the ETCTN Specimen Tracking System (STS) and they must be shipped to the centralized clinical sample repository (currently the EET Biobank).