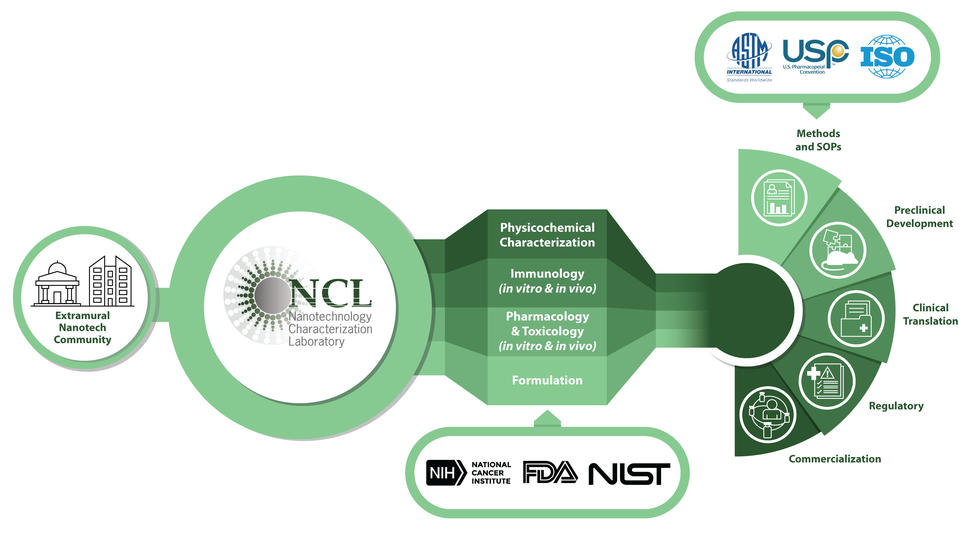
NCL Overview
NCI founded the Nanotechnology Characterization Lab (NCL) in 2004, in collaboration with the U.S. Food and Drug Administration and National Institute of Standards and Technology, as a public–private partnership. It was founded to advance the science needed to expedite the development of promising nanotech therapies and diagnostics. The NCL’s initial mission was to develop an “Assay Cascade” of scientific tests that would help determine the reproducibility, safety, and efficacy of nanomedicines to facilitate regulatory review. The NCL is the only lab with experience testing the wide variety of platforms used in nanomedicine. Since its founding, the NCL has tested more than 500 unique nanomaterials, including almost every type of nanoparticle used in biomedical research and development (e.g., metallic, liposomes, polymers, proteins, micelles, DNA and RNA nanostructures, carbon nanotubes) with nearly every type of active pharmaceutical ingredient (e.g., small molecules, peptides, proteins, nucleic acids, plasmids).
The NCL is located at the Advanced Technology Research Facility in Frederick, Maryland. It has over 11,000 square feet of laboratory and office space. The laboratories are outfitted with two tissue culture rooms, an isotope laboratory, a walk-in cold room, several microscopy rooms, a chromatography and electrophoresis area, a spectroscopy area, a formulation/synthetic lab with chemical fume hoods, multiple customizable research bays, and open floor space for freestanding instrumentation and equipment. The NCL also has direct access to all resources at the Frederick National Laboratory for Cancer Research, including those co-located with the NCL at the Advanced Technology Research Facility.
NCL Annual Report
NCL's 2024 Annual Report highlights the 20th anniversary of the lab's founding, summarizing a symposium held to promote the advancements of nanotechnology over the last two decades, changes in trends seen over the years, as well as recent metrics, publications, and collaborations.
Contact the NCL
Nanotechnology Characterization Laboratory
8560 Progress Drive
Frederick, MD 21701
Phone: 301-846-6939
Fax: 301-846-6399
Email: ncl@mail.nih.gov
NCL Staff
Marina A. Dobrovolskaia, Ph.D., M.B.A., P.M.P.
Dr. Marina Dobrovolskaia is the NCL Co-Director, Director of Operations, and Head of the Immunology Section.
301-228-4935 | marina@mail.nih.gov
Stephan T. Stern, Ph.D., D.A.B.T.
Dr. Stephan Stern is the NCL Co-Director, Director of Research and Development, and Head of the Pharmacology, Toxicology, and Formulation Section.
301-846-6198 | sternstephan@mail.nih.gov
Jeffrey D. Clogston, Ph.D.
Dr. Jeffrey Clogston is the Head of the NCL Physicochemical Characterization Section.
301-846-1388 | clogstonj@mail.nih.gov
Rachael M. Crist, Ph.D.
Dr. Rachael Crist is the Head of the NCL Strategic Client Relations Section.
301-228-4974 | cristr@mail.nih.gov
Other NCL Staff Members
- Edward Cedrone, B.S.
- Matthew Hansen, Ph.D.
- Krishna Kattel, Ph.D.
- Ruvanthi N. Kularatne, Ph.D.
- Barry W. Neun, B.S.
- Timothy M. Potter, B.S.
- Sarah L. Skoczen, M.S.
- Jie Xu, M.S.
NCL Publications