About the OEWG and CTEP Trial Development Deadlines
Operational Efficiency Working Group (OEWG)
In 2008, The NCI's Clinical Trials and Translational Research Advisory Committee (CTAC) established the Operational Efficiency Working Group (OEWG) to identify strategies to reduce the time required for clinical trial development and activation.
Read the Operational Efficiency Working Group (OEWG) Report
Based on the OEWG report presented to CTAC in 2009, CTEP developed an action plan, including implementing development timelines and absolute deadlines for trial activation.
CTEP Trial Development Deadlines, Policies, and Definitions
Trial Development Deadlines
The initial trial development timelines went into effect for applicable Letters of Intent (LOIs) and concepts received starting April 1, 2010. Based on an initial review, the absolute deadlines were shortened in April 2012. The deadline for early phase LOIs was further shortened in 2019. All deadlines are in calendar days.
| Trial Type | Deadline 2010-2012 | Deadline 2012 - Aug 2019 | Current Deadline Sept 2019 - Present |
|---|---|---|---|
| Phase 1 and 2 LOIs | 540 days | 450 days | 400 days |
| Phase 1/2 and 2 Concepts | 540 days | 450 days | 450 days |
| Phase 3 Concepts | 730 days | 540 days | 540 days |
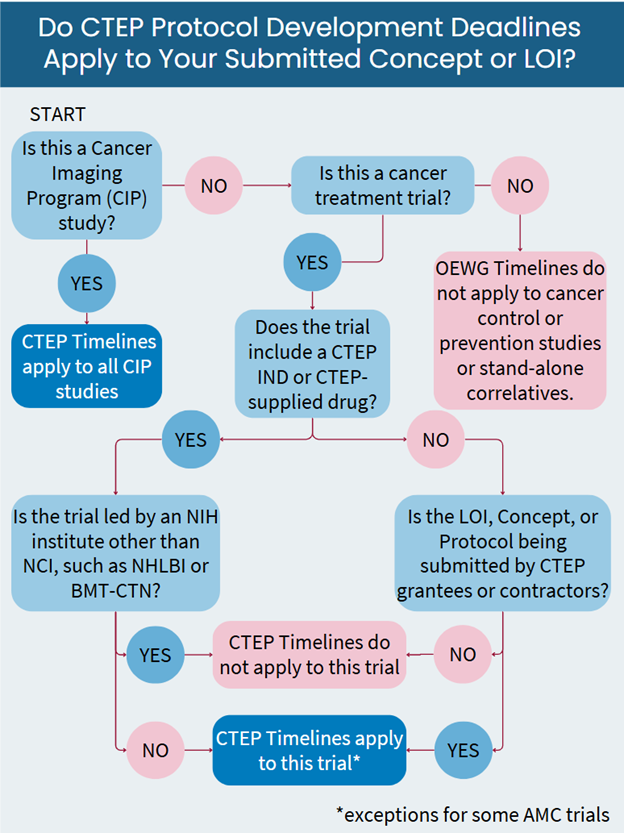
Do The Trial Development Timelines Apply to Your Study?
Not all CTEP-reviewed studies are subject to an OEWG absolute deadline. If you are unsure whether your planned CTEP-reviewed study will be included in OEWG processes, please reach out to the CTEP Protocol Information Office.
Studies with a Trial Development Deadline (Included in OEWG Process) include the following:
- All treatment trials submitted to NCI/CTEP by CTEP/DCTD grantees and contractors, regardless of whether or not CTEP holds the IND.
- All treatment trials submitted to NCI/CTEP in which CTEP holds the IND or is supplying drug, except for trials led by other NIH institutes.
- AIDS Malignancy Clinical Trials Consortium (AMC) treatment trials reviewed by CTEP.
- NCI intramural trials involving a CTEP IND or CTEP-supplied drug.
- All Cancer Imaging Program (CIP) studies (treatment-related and non-treatment-related).
Studies with no Trial Development Deadline (Exempt from OEWG Process) include the following:
- Cancer control and prevention studies.
- NCI intramural studies not involving a CTEP IND or CTEP-supplied drug.
- Clinical Center studies not involving a CTEP IND or CTEP-supplied drug.
- Stand-alone correlative studies (i.e., non-treatment studies), except CIP studies.
- Trials led by other NIH institutes — for example, NHLBI, including BMT-CTN-led trials. Note: Trials that would be included in the CTEP OEWG process per the criteria above (“Inclusions”) and in which BMT-CTN, for example, is a participant are still included in the OEWG process.
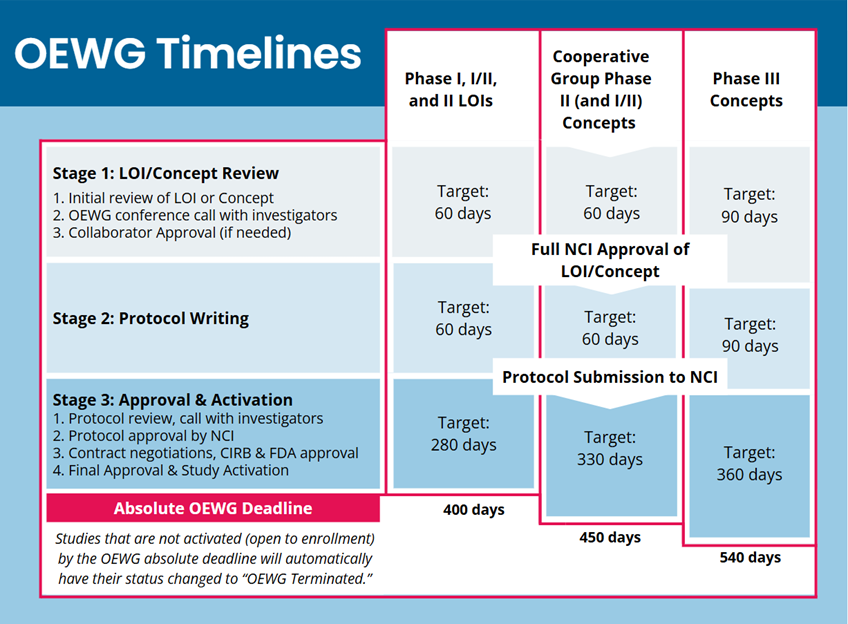
Trial Development Milestones and Timeline Details
CTEP's trial development timelines have three phases. More complex trials (for example, large phase 3 studies) have slightly longer development timelines. All applicable trials must open to enrollment on or before the deadline; those that do not meet the deadline are automatically terminated.
LOI/Concept authoring: initial review to LOI or concept approval.
- The LOI is reviewed by CTEP's Protocol Review Committee; Concepts are reviewed by the relevant Steering Committee.
- CTEP holds a call with investigators to discuss the consensus review; investigators may be asked to submit a revised LOI or concept.
- Approval from both CTEP and the pharmaceutical collaborator are required.
- LOIs may also require biomarker plan approval.
- The target for this phase is 60 days for early phase trials and 90 days for phase 3 trials.
Protocol authoring: LOI approval to initial protocol submission.
- Following approval of the LOI or Concept, the investigators will write and submit the complete study protocol. ETCTN trials will typically work with the Central Protocol Writing Service (CPWS).
- The target for this phase is 60 days for early phase trials and 90 days for phase 3 trials.
Protocol approval and opening to enrollment: protocol submission to initial trial activation.
- Following initial protocol submission and review, CTEP will hold another consensus review call with investigators. There will likely be one or more rounds of revision and re-review.
- Following CTEP approval and collaborator approval, the protocol and associated documents will be routed to the NCI Central IRB and to the FDA for review.
- All contract negotiations, study site finalization, CTSU set-up, and trial activation activities are completed.
- The trial must open to patient enrollment on or before the Trial Development Deadline (OEWG Deadline).