Operations and Management
About
The Chemical Biology Consortium is the Discovery engine of the NExT Program. All applications for Discovery stage projects that are accepted into the NExT Program are fully operated through the CBC, with management and oversight being provided through the Frederick National Laboratory for Cancer Research staff. The Operations are driven through funding awards derived from a competitive source selection process that stems from a project plan facilitated by both NCI and FNL staff in collaboration with principal investigators on the project. Resource funding is awarded to centers and is based on external reviewer feedback and senior leadership approval in specific performance periods, with an expectation that projects will meet predesignated milestones and go/no-go decision points that are established by the project teams. Projects that continue to meet milestones are typically provided resourcing through the CBC center subcontracts to meet future periods of performance and milestones.
CBC Membership
Since 2009, the NCI has held an open competition for membership into the Chemical Biology Consortium (CBC) on a 5-7 year basis. Now in the third phase, the CBC has taken measures to maintain optimal mixtures of research and development centers, including designated cancer centers and research centers of excellence, that cover the breadth of resource needs to advance a discovery stage project from Oncology Target Validation through exploratory toxicology studies in preparation for IND filing of developmental candidate compounds. CBC members have the opportunity to meet several times a year, as part of a steering committee, to provide guidance to new and existing projects. In addition, CBC members are given priority consideration during source selection process for work assignment activities requested from a given project.
Collaborations
At times, there may be a need for a resource or area of expertise that falls outside the scope of the Chemical Biology Consortium. In such cases, the NExT Program looks to other opportunities outside the consortium to bridge any gaps and ensure the needs of the project are fully met. If you have a unique capability within the drug discovery stages and would like to be considered for consideration as needs arise, please contact the NExT Program at NCI NExT Info Mail (NCINExTinfo@mail.nih.gov).
An additional mechanism for investigators or companies to collaborate involves applying to NExT for the development of a therapeutic agent. NExT applicants with unique capabilities around a given cancer indication or agent type are accepted into the CBC and can be granted funding to provide a specific resource that impacts the advancement of a project. For example, if there is a unique assay and/or endpoint measurement that is developed in a PIs laboratory for a given target that demonstrates specificity and on-target efficacy readout deemed critical to a compound's selection or advancement, the PI may be asked to become a member of the CBC to contribute this capability to the project, and potentially future projects, if the capability is useful as a fit for purpose assay.
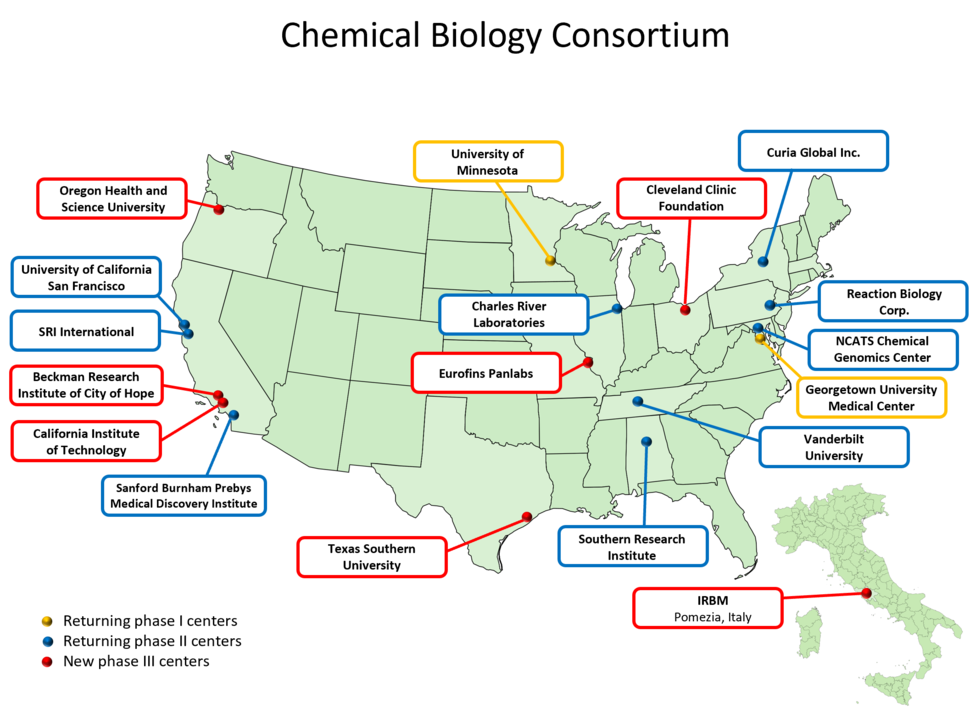
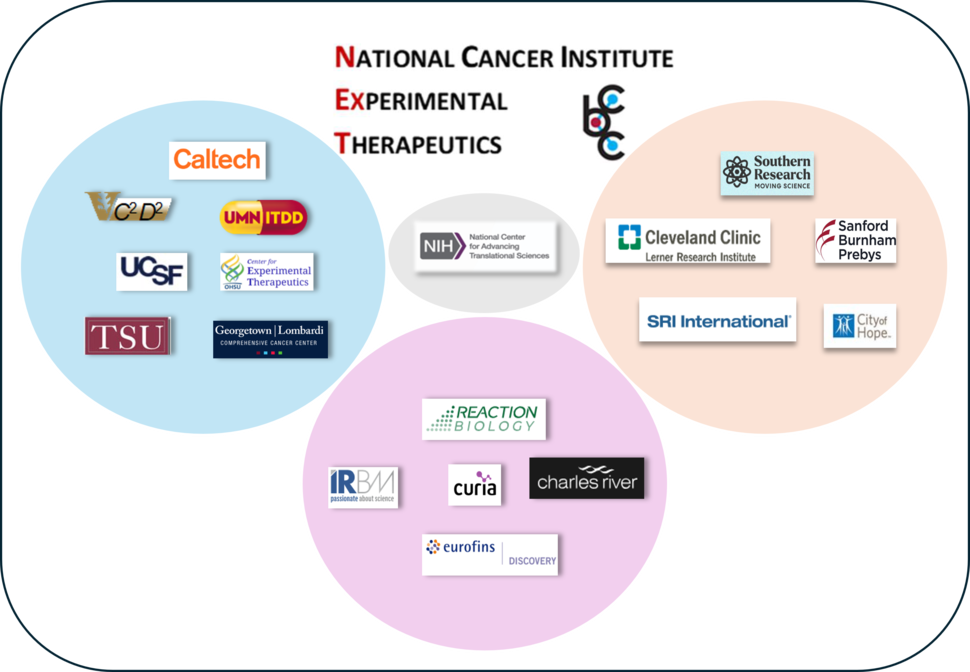
Phase III participating CBC Centers
Non-Member Participants
The NExT Discovery portfolio will occasionally need to tap into non-CBC participants to complete critical path activities. Some of the participants that have provided project assistance are listed below, and include government and CRO sources:
Government
- Clinical Pharmacodynamics - Biomarkers Program (FNL)
- Biological Testing Branch (NCI/DTP)
- Drug Synthesis and Chemistry Branch (NCI/DTP)