Toxicology and Pharmacology Branch (TPB)
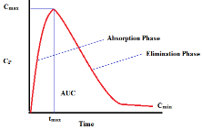
Preclinical toxicology and pharmacology are required for decision making throughout drug discovery and development and for IND filing for clinical trials. Toxicological and pharmacological data can inform clinical trial design, such as determination of maximum tolerated dose, dose-limiting toxicities, and starting dose. With appropriate characterization, in most cases, safe operating parameters can be established for human clinical trials.
Ultimate Goal
The ultimate goal is to identify and characterize the toxicologic profile and pharmacokinetic properties of potential drug candidates and to support an Investigational New Agent Application (IND) or a Drug Master File (DMF) with the USFDA.
Drug Discovery and Development Process
Exploratory Screen Development →Screening/Designed Synthesis →Lead Development →Candidate Seeking →Clinical Candidate