Synthesis of Silvestrol
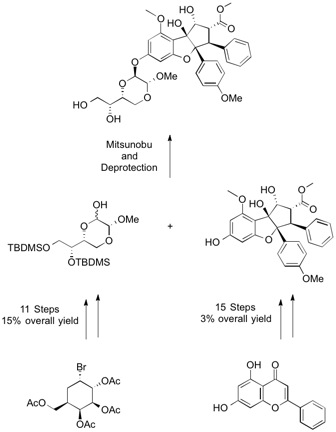
In support of the preclinical development of silvestrol at NCI, DSCB has completed the preparation of multigram quantities of the natural product silvestrol through total synthesis, using previously reported methodology1 as the template for the scale-up work.
1. (a) Adams, Tim E, El Sous, Mariana, Hawkins, Bill C, Hirner, Sebastian, Holloway, Georgina, Khoo, Mui Ling, Owen, David J, Savage, G Paul, Scammells, Peter J, Rizzacasa, Mark A; Total synthesis of the potent anticancer Aglaia metabolites (-)-silvestrol and (-)-episilvestrol and the active analogue (-)-4'-desmethoxyepisilvestrol, J. Am. Chem. Soc. 2009, 131, 1607-16. (b) Liu, Tao, Nair, Somarajan J, Lescarbeau, André, Belani, Jitendra, Peluso, Stéphane, Conley, James, Tillotson, Bonnie, O'Hearn, Patrick, Smith, Sherri, Slocum, Kelly, West, Kip, Helble, Joseph, Douglas, Mark, Bahadoor, Adilah, Ali, Janid, McGovern, Karen, Fritz, Christian, Palombella, Vito J, Wylie, Andrew, Castro, Alfredo C, Tremblay, Martin R; Synthetic silvestrol analogues as potent and selective protein synthesis inhibitors, J. Med. Chem. 2012, 55, 8859-78. (c) Gaudouin, B, Jones II, G, Porco Jr, J; A biomimetic approach to the rocaglamides employing photogeneration of oxidopyryliums derived from 3-hydroxyflavones, J. Am. Chem. Soc. 2004, 126, 13260-13621.
Preclinical development of T-dCyd
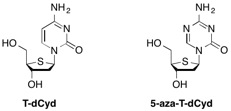
DNMT1 is a maintenance methyltransferase that contributes to the hypermethylation and silencing of tumor suppressor genes. In addition, DNMT1 also has roles independent of its methyltransferase activity and its knockout causes decreases in cell viability that are preceded by events consistent with activation of DNA damage response. 4'-Thio-2'-deoxycytidine (T-dCyd) is an agent that depletes DNMT1 both in vitro and in vivo in tumor cells. In non-small cell lung cancer NCI-H23 xenograft studies, treatment with T-dCyd resulted in the inhibition of tumor growth with concomitant DNMT1 depletion at well tolerated doses.1 Given the significant compound supply needs consistent with preclinical development, DSCB undertook the development of an efficient process for the synthesis of T-dCyd2 and a close analog, 5-aza-T-dCyd3.
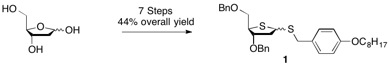
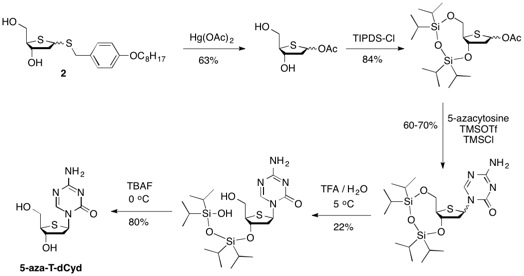
The syntheses of T-dCyd and 5-aza-T-dCyd make use of the dibenzyl thiofuranose derivative 1 which is readily prepared in 7 steps (44% overall yield) starting with 2-deoxy-D-ribose.
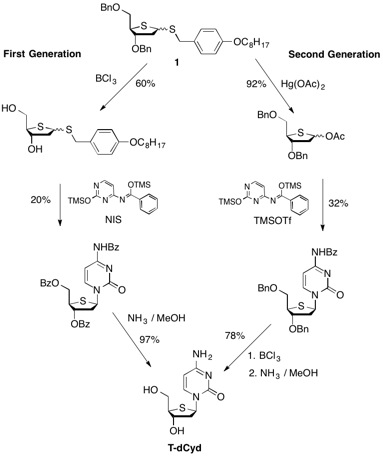
The first-generation synthesis of T-dCyd involved the initial deprotection of the dibenzyl thiofuanose 1 with boron trichloride to produce diol 2. Reprotection with benzoyl chloride followed by reaction with 4'-benzoylcytosine and N-iodosuccinimide produced a 60% yield of a 6:5 mixture of alpha and beta isomers. Following column chromatography and methanol crystallization, a 20% overall yield of purified beta isomer 3 was obtained. Global deprotection with methanolic ammonia cleanly afforded T-dCyd in 97% yield. This route (which proceeded in 11% overall yield) was hampered with regards to throughput because the initial benzyl ether deprotection step could not be performed on a scale greater than 90 grams without significant loss in yield.
The synthesis of 5-aza-T-dCyd starting from diol 2 involved two key steps after conversion to the cyclic tetraisopropyldisilyloxy derivative 6, including 1) reaction of 6 with 5-azacytosine to give a 60-70% yield of a mixture of alpha and beta coupled isomers and 2) the selective deprotection of the primary silyloxy group which allowed for the chromatographic isolation of the purified beta isomer 8 in 22% overall yield from 6. The removal of the remaining silyloxy group with tetra-butyl ammonium fluoride produced 5-aza-T-dCyd in 80% yield.
1. Kinders, R., Hollingshead, M., Thotassery, J., Parker, W., Pfister, T., Morris, J., Anderson, L., Tomaszewski, J., Collins, J., Doroshow, J.; Pre-clinical development of 4'-thio-2'-deoxycytidine (T-dCyd) as a DNA-demethylating agent for use in treating solid tissue tumors. Poster presentation at the AACR Meeting in San Diego, CA, April 9, 2014.
2. (a) Dyson, Michael R., Coe, Paul L., Walker, Richard T., An improved synthesis of benzyl 3,5-di-O-benzyl-2-deoxy-1,4-dithio-D-erythro-pentofuranoside, an intermediate in the synthesis of 4'-thionucleosides, Carbohydrate Research, 216 (1991) 237-248. (b) Jun-ya Hasegawa, Masahiro Hamamda, Tetsuo Miyamato, Kiyoharu Nishide, Tetsuya Kajimoto, Jin-ichi Uenishi, and Manabe Node, The application of phenylmethanethiol and benzenethiol derivatives as odorless organosulfur reagents in the synthesis of thiosugars and thioglycosides, Carbohydrate Research, 340, (2005) 2360-2368. (c) Secrist, John A., III; Tiwari, Kamal N.; Riordan, James M.; Montgomery, John A., Synthesis and Biological Activity of 2'-Deoxy-4'-thio Pyrimidine Nucleosides, Journal of Medicinal Chemistry (1991), 34(8), 2361-6. (d) Secrist, John A., III; Parker, William B.; Tiwai, Kamal N.; Messini, Lea; Shaddix, Sue C.; Rose, Lucy M.; Lee Bennett, L., Jr.; Montgomery, John A., The Synthesis and Biological Activity of certain 4'-thionucleosides, Nucleosides & Nucleotides (1995), 14(3-5), 675-86.
3. Kamal N. Tiwari, Loredana Cappellacci, John A. Montgomery, and John Secrist, Syntheis and Anticancer Activity of Some Novel 5-Azacytosine Nucleosides, Nucleosides, Nucleotides, and Nucleic Acids, (2003), 22 (12), 2161-2170.