Additional Supported Validated Biomarker Assays
DCTD provides training and key reagent support to the cancer research community for the following list of additional supported validated biomarker assays.
We developed the assays employing quality-controlled reagents, standards, and controls and the assay and supporting specimen handling SOPs ensuring inter-operator, inter-run, and inter-site reproducibility. Rigorous methodologies and reference materials result in accurate and reproducible evaluation of drug effect in heterogeneous clinical specimens.
ELISA Immunoassays
Intact MET, Dual Phospho-Y1234/Y1235 and Phospho-Y1356 Immunoassays
Intact MET Solid Tumor Assessment
The Intact MET Immunoassay has been developed to measure the effect of anticancer agents on the levels of intact MET in tumor biopsy samples, irrespective of the MET phosphorylation levels. The amount of intact MET measured will serve as a denominator reading to determine the fraction of MET phosphorylated at critical sites.
- Specimen Collection
- Sample Preparation
- Immunoassay SOP
- Data Analysis, Quality Control, and Reporting SOP
Dual Phospho-Y1234/1235 MET Solid Tumor Assessment
The Dual Phospho-Y1234/Y1235 MET Immunoassay has been developed to measure the effects of anticancer agents on pMET levels in MET-overexpressed/amplified disease conditions in tumor biopsy samples. The amount of intact MET measured in Intact MET Immunoassay will serve as a denominator reading to determine the fraction of dual phospho-Y1234/Y1235 MET in the samples.
- Specimen Collection
- Sample Preparation
- Immunoassay SOP
- Data Analysis, Quality Control, and Reporting SOP
Phospho-Y1356 MET Solid Tumor Assessment
The Phospho-Y1356 MET Immunoassay has been developed to measure the effect of anticancer agents on pMET levels in MET-overexpressed/amplified disease conditions in tumor biopsy samples. The amount of intact MET measured in Intact MET Immunoassay will serve as a denominator reading to determine the fraction of phospho-Y1356 MET in the samples.
- Specimen Collection
- Sample Preparation
- Immunoassay SOP
- Data Analysis, Quality Control, and Reporting SOP
NCI Publications
- Molecular Pharmacodynamics-Guided Scheduling of Biologically Effective Doses: A Drug Development Paradigm Applied to MET Tyrosine Kinase Inhibitors.
- Pharmacodynamic Response of the MET/HGF Receptor to Small-Molecule Tyrosine Kinase Inhibitors Examined with Validated, Fit-for-Clinic Immunoassays.
- Combination therapy with pazopanib and tivantinib modulates VEGF and c-MET levels in refractory advanced solid tumors.
- Effective implementation of novel MET pharmacodynamic assays in translational studies.
Poly-Adenosyl Ribose (PAR) Immunoassay
The PAR Immunoassay has been developed to measure the effect of PARP inhibitors and/or chemotherapeutic agents on PAR levels in a variety of biospecimen types, including peripheral blood mononuclear cells (PBMCs) and tumor biopsies.
Solid Tumor Assessment
- Specimen Collection
- Sample Preparation
- Immunoassay SOP
- Data Analysis, Quality Control, and Reporting SOP
Liquid Tissue Assessment
- Specimen Collection
- Sample Preparation
- Immunoassay SOP
- Data Analysis, Quality Control, and Reporting SOP
NCI Publications
- A phase I combination study of olaparib with cisplatin and gemcitabine in adults with solid tumors.
- A phase I study of veliparib in combination with metronomic cyclophosphamide in adults with refractory solid tumors and lymphomas.
- Modeling pharmacodynamic response to the poly(ADP-ribose) polymerase inhibitor ABT-888 in human peripheral blood mononuclear cells.
- Phase I study of PARP inhibitor ABT-888 in combination with topotecan in adults with refractory solid tumors and lymphomas.
- Histone γH2AX and poly(ADP-ribose) as clinical pharmacodynamic biomarkers.
Immunofluorescence Assays
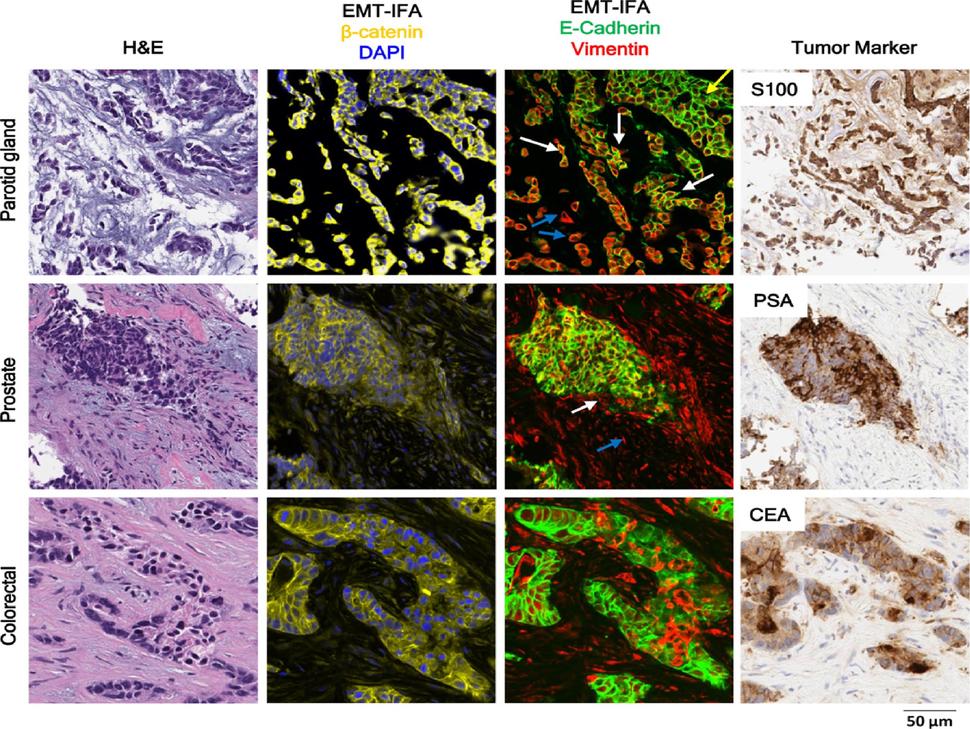
Epithelial-Mesenchymal Transition (EMT) Panel Immunofluorescence Assay
The EMT Panel IFA was developed to evaluate the epithelial/mesenchymal phenotype of tumor tissue sections. The assay uses monoclonal antibodies to EMT biomarkers, including E-Cadherin, Vimentin and β-Catenin, directly conjugated to various Alexa Fluor dyes as reporters for immunostaining.
Assay SOPs
NCI Publication
Contact
PDinfo@mail.nih.gov (questions, training requests or comments)
Additional Publications
Find Additional Publications for the Poly-Adenosyl Ribose (PAR) Immunoassay here.