Historical Biomarker Assays
DCTD-supported Pharmacodynamic Biomarkers Program offers historical biomarker assays. Unlike other validated biomarker assays, these published assays do not have a formal training program or qualified reagent supply; however, consultation may be requested.
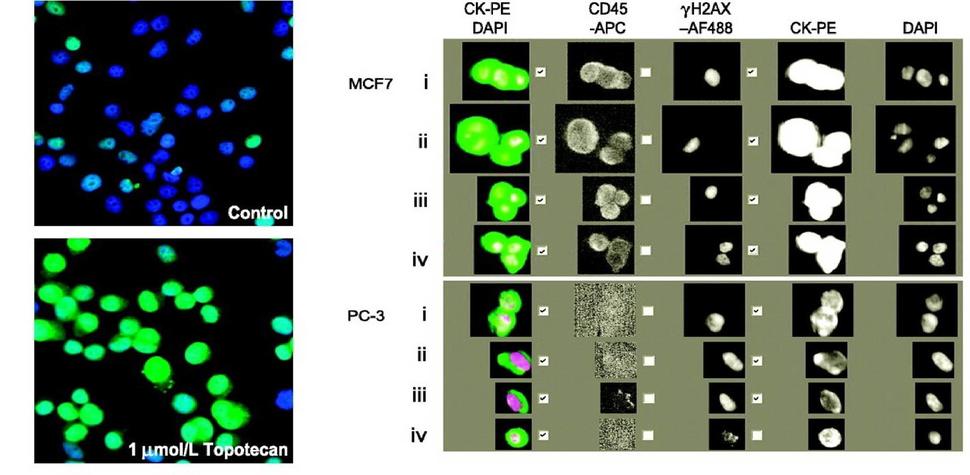
γH2AX, pNBS1, and β-Catenin Multiplex Immunofluorescence Assay
The γH2AX, pNBS1 IFA with β-Catenin segmentation is an immunofluorescence-based assay developed to quantify γH2AX and pNBS1 using β-Catenin staining to segment tumor from non-tumor to precisely limit quantitation of these DNA damage response markers to the tumor cells within a tumor biopsy section.
Assay SOPs
Specimen Collection:
- Sample Preparation
- Assay SOP
- Image Capture
- Image and Data Analysis
NCI Publications
Analysis of Circulating Tumor Cells Using the CellSearch System
Biomarker Assessment
- CellSave Tube Blood Collection and Shipping Instructions
Antibody Qualification and Laboratory Proficiency Testing SOPs:
- Assay SOP
NCI Publications
- A phase I study of veliparib in combination with metronomic cyclophosphamide in adults with refractory solid tumors and lymphomas.
- Phase I study of PARP inhibitor ABT-888 in combination with topotecan in adults with refractory solid tumors and lymphomas.
- Histone γH2AX and poly(ADP-ribose) as clinical pharmacodynamic biomarkers.
- Monitoring drug-induced γH2AX as a pharmacodynamic biomarker in individual circulating tumor cells.
- Use of circulating tumor cells to monitor pharmacodynamic responses of patients in early stage clinical trials.
Immunoassays (ELISA)
Hypoxia Inducible Factor 1-alpha, ELISA
Solid Tumor Assessment
- Specimen Collection
- Sample Preparation
- Immunoassay SOP
- Data Analysis, Quality Control, and Reporting SOP
NCI Publications
- Validation of a hypoxia-inducible factor-1 alpha specimen collection procedure and quantitative enzyme-linked immunosorbent assay in solid tumor tissues.
- Pilot trial of EZN-2968, an antisense oligonucleotide inhibitor of hypoxia-inducible factor-1 alpha (HIF-1α), in patients with refractory solid tumors.
- Weekly EZN-2208 (PEGylated SN-38) in combination with bevacizumab in patients with refractory solid tumors.
Topoisomerase I (Top1) Immunoassay
Solid Tumor Assessment
- Specimen Collection
- Sample Preparation
- Immunoassay SOP
- Data Analysis, Quality Control, and Reporting SOP
Liquid Tissue Assessment
- Specimen Collection
- Sample Preparation
- Immunoassay SOP
- Data Analysis, Quality Control, and Reporting SOP
NCI Publications
- Development and validation of an immunoassay for quantification of topoisomerase I in solid tumor tissues.
- Development of a validated immunofluorescence assay for γH2AX as a pharmacodynamic marker of topoisomerase I inhibitor activity.
- Topoisomerase I levels in the NCI-60 cancer cell line panel determined by validated ELISA and microarray analysis and correlation with indenoisoquinoline sensitivity.
Contact
PDinfo@mail.nih.gov (questions, training requests, or comments)