Stable Isotope Tracer Ultrafiltration Assay (SITUA)
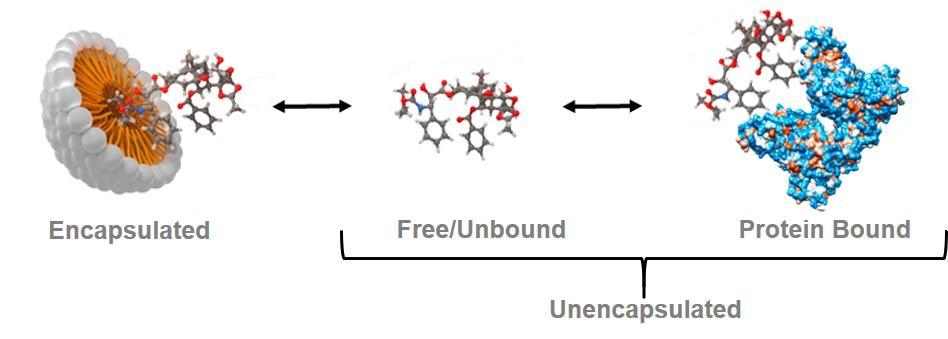
The complexity of nanomedicine drug formulations poses unique scientific challenges. In contrast to conventional small molecule formulations, the active pharmaceutical ingredient (API) in a systemically administered nanomedicine formulation exists in several forms:
- nanomedicine-encapsulated
- unencapsulated, free/unbound
- unencapsulated, protein-bound
While the free/unbound form is considered the only biologically active form of the active pharmaceutical ingredient, all three fractions are important in characterizing a nanomedicine’s pharmacokinetics—especially in evaluation of bioequivalence (pharmacokinetic similarity). Existing methods to measure these various nanomedicine fractions (e.g., solid phase extraction, conventional ultrafiltration) are not ideal due to a variety of shortcomings. The Nanotechnology Characterization Laboratory (NCL) has developed a novel bioanalytical technique, called the stable isotope tracer ultrafiltration assay (SITUA), to fractionate the various subpopulations of a nanomedicine in plasma, in an effort to fulfill this unmet need and facilitate regulatory review of generic nanomedicines or nanosimilars.
The assay uses a stable isotopically labeled version of the nanomedicine-encapsulated drug and an ultrafiltration technique. When spiked into plasma or other matrix, the stable isotope labeled drug equilibrates with protein and nanomedicine identical to the unlabeled, normoisotopic drug. Therefore, the ultrafiltrate fraction of the isotopically labeled drug represents a reliable measurement of the free drug fraction. The plasma protein-bound, unencapsulated, and encapsulated nanomedicine fractions can then be easily calculated. More details on the SITUA can be found in the following publications.
Skoczen SL, Snapp KS, Crist RM, Kozak D, Jiang X, Liu H, Stern ST. Distinguishing pharmacokinetics of marketed nanomedicine formulations using a stable isotope tracer assay. ACS Pharmacol Transl Sci 2020; 3(3); 547-558. [PubMed Abstract]
Skoczen SL, Stern ST. Improved ultrafiltration method to measure drug release from nanomedicines utilizing a stable isotope tracer, in Characterization of nanoparticles intended for drug delivery, S. McNeil, Editor. Methods Mol Biol. 2018; 1628, 2018, 223-239. [PubMed Abstract]
Skoczen S, McNeil SE, and Stern ST. Stable isotope method to measure drug release from nanomedicines. J Control Release 2015; 220(Pt A); 169-174. [PubMed Abstract]
Available Services
The NCL currently has two Technical Services available, both based on the Stable Isotope Tracer Ultrafiltration Assay (SITUA).
NCL-01: An in vitro drug release study in human plasma using the SITUA. The assay will provide concentrations of encapsulated, free/unbound, and protein-bound drug and is ideal for formulation optimization and lot release.
NCL-02: A pharmacokinetic study in rats using the SITUA. Blood samples will be collected at various timepoints for analysis of encapsulated, free/unbound, and protein-bound drug concentrations, as well as noncompartmental pharmacokinetic analysis to complement preclinical bioequivalence evaluation.
Hear Dr. Stephan Stern talk about the SITUA with Dr. Vladimir Popov, Director of the Frederick National Lab's Partnership Development Office.
Purchasing a Technical Service
Interested parties are encouraged to first email the NCL (ncl@mail.nih.gov). A preliminary discussion will help the NCL gain an understanding of your technology, nanoformulation properties, and how the selected service is expected to aid your developmental path. In addition, we’ll be able to address any questions you have about the assay, sample requirements, or time lines.
After this initial discussion, the NCL will put you in contact with the Partnership Development Office for the Frederick National Lab. They will guide you through the necessary steps for purchasing the Technical Service, including execution of the Technical Services Agreement contract. Once the agreement is executed, payment must be made in full prior to shipping samples.
Questions
For scientific questions related to the assays, please email the NCL (ncl@mail.nih.gov). We’re happy to discuss the feasibility of the study and sample requirements prior to initiation of the contract.