The Mission of the Radiation Research Program
The Radiation Research Program (RRP) supports the basic, translational and clinical research. It is a part of an ongoing effort to stimulate research in radiotherapy and radiation biology, in the Division of Cancer Treatment and Diagnosis (DCTD) by:
- Providing expertise to investigators and potential grantees who perform cutting-edge research with radiation and other forms of energy;
- Helping to lead the radiotherapy research community in establishing priorities for the future direction of radiation research, including targeted funding initiatives and interagency collaboration;
- Developing and promoting collaborative efforts among extramural investigators for both pre-clinical studies and clinical trials;
- Developing unique models and capabilities to help communities in the United States and worldwide to access cancer clinical trials;
- Developing, managing, and evaluating the NCI radiation research portfolio;
- Advising the NCI-funded clinical trials groups and the Cancer Therapy Evaluation Program (CTEP) regarding scientific priorities and quality assurance in clinical studies with radiotherapy;
- Providing guidance to extramural investigators and collaborating with DCTD experts to develop novel combined modality therapy;
- Serving as the NCI’s liaison and advisor on the mitigation of radiation injury to normal tissue and the development of biomarkers for radiation injury in programs addressing radiological and nuclear terrorism in the National Institute of Allergy and Infectious Diseases (NIAID) and the Office of the Assistant Secretary for Preparedness and Response within the Department of Health and Human Services.
RRP coordinates its activities with other radiation research efforts at NCI:
- Division of Cancer Biology
- SBIR Development Center
- Division of Cancer Control and Population Sciences
- Cancer Center for Research — Radiation Biology Branch
- Cancer Center for Research — Radiation Oncology Branch
- Division of Cancer Epidemiology and Genetics
- The National Institutes of Health (NIH) and other federal agencies
- National and International research organizations.
The Radiation Research Program serves as a focal point for extramural investigators seeking grants and contracts to support clinical, pre-clinical, and basic mechanistic studies related to radiation oncology, radiobiology, and cancer biology research.
About the Acting Associate Director
Paula M Jacobs, Ph.D.
Dr. Jacobs received her undergraduate degree at the Massachusetts Institute of Technology and graduate degrees at Tufts University and Northeastern University. Her post-doctoral training was at Northeastern University, Massachusetts Institute of Technology, and Peter Bent Brigham Hospital/Harvard Medical School.
In the Radiation Research Program she oversees extramural grants in radiation research, including external radiation therapy, radiation biology and radiopharmaceuticals for targeted radiation therapy.
She is a member of the FDA Medical Imaging Drugs Advisory Committee and of the steering/advisory committees for the Integrated Canine Data Commons, the Small Animal Imaging Program at Frederick National Laboratory for Cancer Research and the NIBIB Medical Imaging Data Resource Center. She directs imaging studies in mice of patient derived xenograft cancer models.
Previously, she was Associate Director, Cancer Imaging Program, overseeing extramural grants in molecular imaging, nanotechnology, image guided interventions, imaging technology, and clinical trials in imaging. She focused on lowering the logistical and regulatory barriers to investigational use of PET radiopharmaceuticals, developed the Cancer Imaging Archive, a publicly available medical image archive with associated clinical meta-data for machine learning, and encouraged standardization of imaging techniques and quantitative image methods.
She joined the NCI after 30 years of diverse experience in the pharmaceutical and medical device industries. Her last industrial position was Vice President, AMAG Pharmaceuticals (now Covis Pharma) developing ultrasmall superparamagnetic iron oxide nanoparticles for MRI imaging and therapeutic iron drugs.
She has published in organic chemistry, inorganic chemistry, magnetic resonance imaging, PET imaging, regulatory affairs, neuro-oncology, and nephrology.
Organizational Structure
The Radiation Research Program (RRP) is one of eight major programs in the Division of Cancer Treatment and Diagnosis of the National Cancer Institute.
The Radiation Research Program is divided into two highly collaboratively interactive branches: Clinical Radiation Oncology Branch (CROB) and Radiotherapy Development Branch (RDB). Program Officers in RRP often collaborate with each other and with Program Officers in other Programs, Divisions, and Institute.
The Clinical Radiation Oncology Branch plans, develops, and executes a program in clinical radiation oncology, therapeutic nuclear medicine and medical physics research, with radiation oncology broadly defined to include radiation used alone and in combination with chemical, immunological and biological agents, hyperthermia and radiation modifiers. It reviews all clinical trials involving radiation therapy, in protocol review for NCI's Clinical Trials Network (NCTN), the Experimental Therapeutics Clinical Trials Network (ETCTN) and Community Oncology Research Program (NCORP).
The Radiotherapy Development Branch fosters an improved understanding of the therapeutic utility of radiation and other forms of energy. It uses a variety of existing funding mechanisms and novel collaborations to support basic, preclinical, and translational research related to improving the outcomes of radiation and drug combination treatments to cancer patients, safety, efficacy, and quality-of-life. It is a focal point for advancing basic and preclinical research in Radiation Oncology within NIH and NCI.
Funding Information
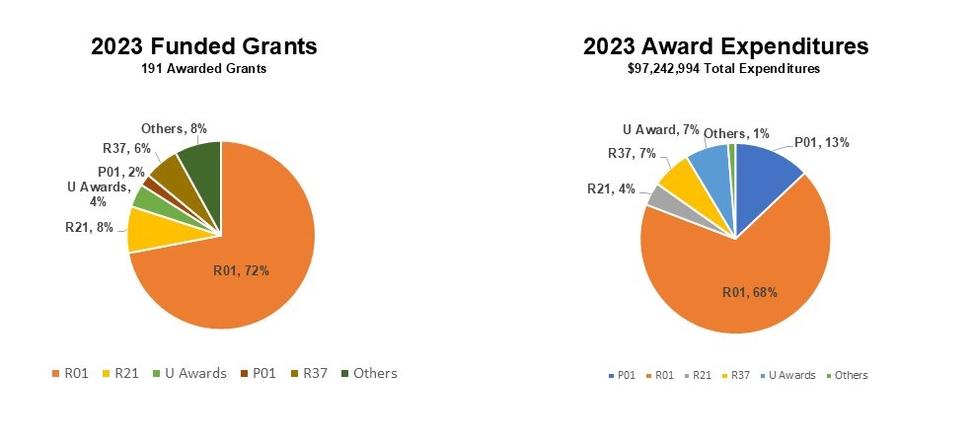
The primary responsibility of RRP is to the grantees and contractors of NCI and NIH awards. In fiscal year 2023, RRP administered 191 awarded grants, of which R01s (72%) made most of the awarded grants. A total of $97,242, 994 were incurred in total expenditures for 2023, of which 68% came from R01 expenditures and 13% from P01 grants. These numbers are illustrated in the pie charts below.
RRP Grants Award Distribution and Expenditures 2023
For useful grant-related information including general guidelines, application forms, funding opportunities and human subjects research guidelines, please visit grants.gov. For Small Business Innovation Research (SBIR) Funding Opportunities which include solicitations for development of radiation-related technologies please visit sbir.cancer.gov.
Precision Approaches in Radiation Synthetic Combinations (PAIRS, R01 Clinical Trial Optional)
Precision Approaches in Radiation Synthetic Combinations (PAIRS, R21 Clinical Trial Optional)
Systematic Testing of Radionuclides in Preclinical Experiments (STRIPE) (R01 Clinical Trial Not Allowed)
Systematic Testing of Radionuclides in Preclinical Experiments (STRIPE) (R21 Clinical Trial Not Allowed)