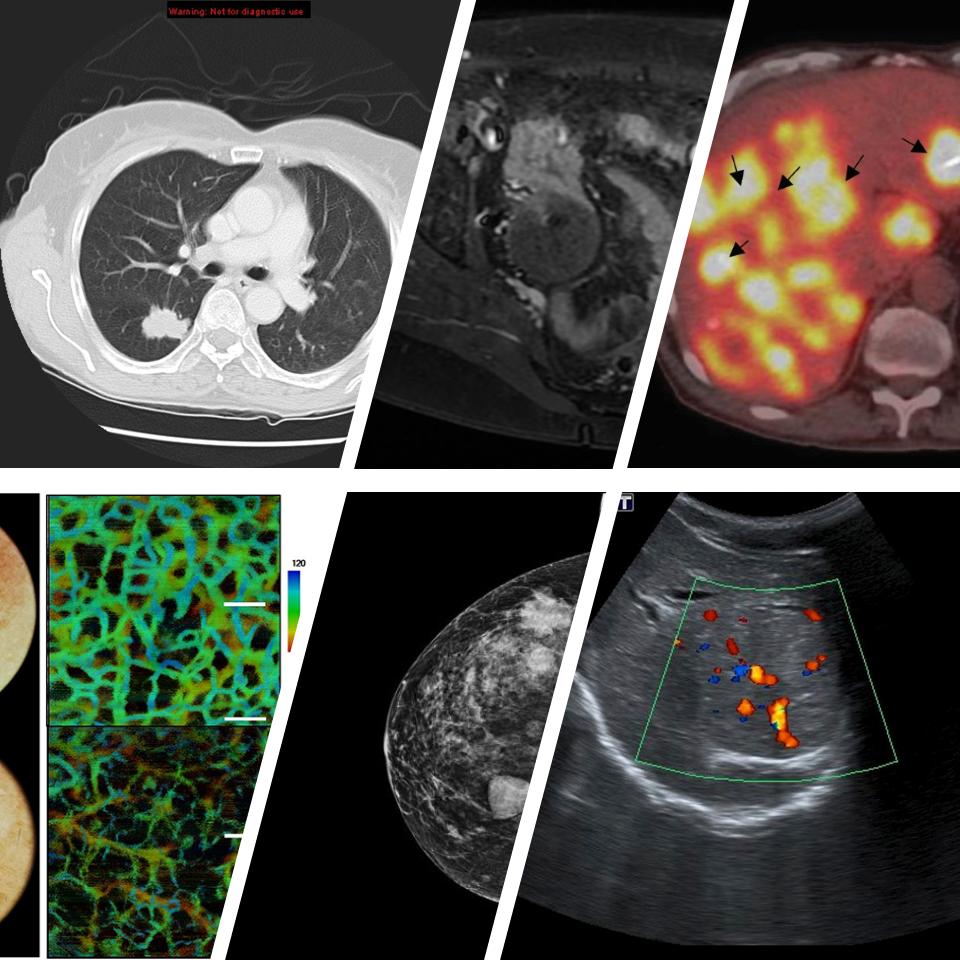
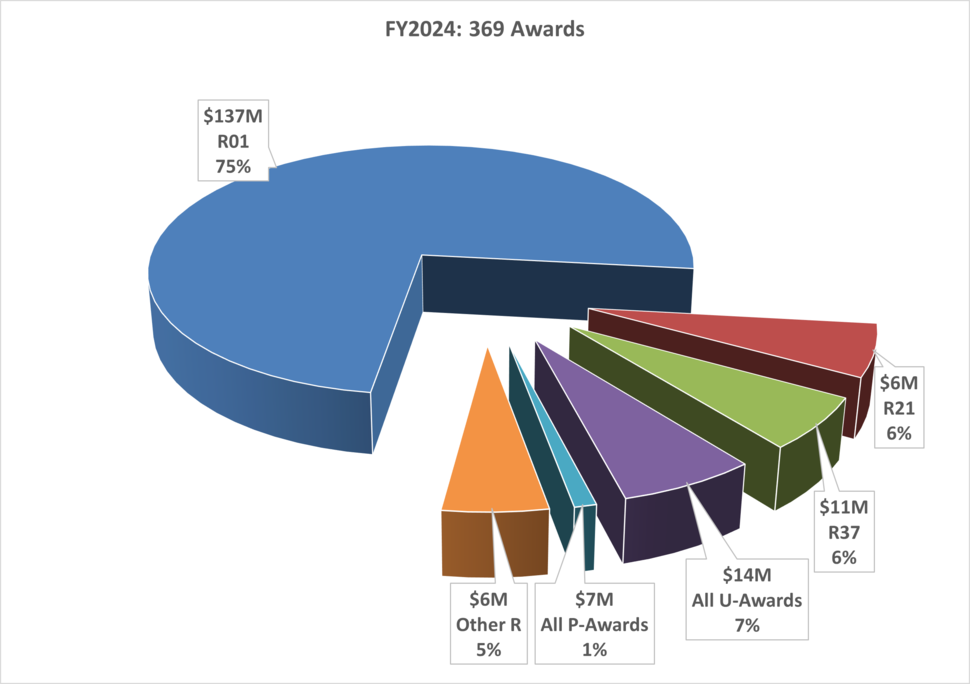
Mission, Vision and Goals
Mission
The NCI Cancer Imaging Program fosters advances in in vivo medical imaging sciences through support of basic and applied research in cancer imaging as well as promotion of imaging in clinical trials in order to gain greater understanding of the pathways of cancer biology for the benefit of cancer patients and people at cancer risk.
Vision and Goals
Cancer Imaging: visualize the problem and direct the solution.
- Infrastructure and programs to support the discovery and development of molecular imaging for cancer care and the understanding of cancer biology.
- A set of imaging methods validated as cancer biomarkers, some of which are surrogate endpoints.
- Infrastructure and programs to support the discovery, development and delivery of image-dependent interventions for cancer and pre-cancer.
- An implemented infrastructure based on standardized models for the design and conduct of clinical trials of, or using, imaging and image-guided interventions.
- Accelerated development and delivery of integrated imaging systems and methods for cancer care and research.
- A critical role in NIH and NCI activities in emerging technologies, such as nanotechnology, proteomics, and high-throughput screening technologies.
- An implemented informatics infrastructure to optimize the value of cancer imaging data.
- A strategy of imaging science and methods to detect, treat and monitor response to therapy in pre-cancer.
Cancer Imaging Program History
The National Cancer Institute (NCI) established the Diagnostic Imaging Program (DIP) in October, 1996. David Bragg, M.D., the first acting director, led the DIP with two full-time staff members. Daniel C. Sullivan, M.D., became the first full-time director in September, 1997. The name of the program has changed twice during this time, from the DIP to the Biomedical Imaging Program in 2001, to finally to the Cancer Imaging Program (CIP) in 2003. This name more clearly reflects the role of the program, both to NCI and to the public. James Tatum, MD, was appointed to direct the program in 2007, followed by Paula Jacobs, Ph.D., in 2011. From 2012-2024, Dr. Janet Eary, M.D., was the acting program director.
The staff members and administered grants are divided among five branches: the Clinical Trials Branch, the Molecular Imaging Branch, the Image-Guided Intervention Branch, the Imaging Technology Development Branch and the Nanodelivery Systems and Devices Branch.
Organizational Structure
The Cancer Imaging Program (CIP) is one of eight major Programs in the Division of Cancer Treatment and Diagnosis (DCTD) of the National Cancer Institute.
The Cancer Imaging Program consists of 5 branches. Each branch has a Branch Chief and associated Program Directors. Because of the cross-cutting nature of many cancer imaging activities, CIP staff often collaborate across branches and are not strictly confined to activities monitored by the branch with which they are formally associated.
- Molecular Imaging Branch: To encourage the development of molecular imaging from basic discovery of methods and agents to their development as preclinical tools and into clinical use in the service of diagnosis and therapy of cancer patients and those at risk.
- Imaging Technology Branch: To encourage and nurture the development and translation of medical imaging instrumentation and technology for the diagnosis and therapy of cancer.
- Clinical Trials Branch: To promote the study of the efficacy of diagnostic imaging techniques through clinical trials in order to diagnose and treat cancer more effectively and at an earlier stage.
- Image-Guided Intervention Branch: To promote the use of imaging techniques in the performance of diagnostic and therapeutic procedures for the diagnosis and treatment of cancer.
- Nanodelivery Systems and Devices Branch: To promote the use of nanotechnologies in fundamental studies of cancer biology and in the development of new cancer interventions related to in vitro diagnosis, imaging and treatment of cancer.
CIP Organization Chart
About the Acting Associate Director
Lalitha K. Shankar, M.D., Ph.D.
Acting Associate Director
Branch Chief: Clinical Trials Branch
Lalitha K. Shankar, M.D., Ph.D., is at the Cancer Imaging Program at the National Cancer Institute, at the National Institutes of Health in Bethesda, MD. Since joining NCI in 2003, she has served as an Advisor to the Associate Director of the Division of Cancer Treatment and Diagnosis. She is the Chief of the Clinical Trials Branch at the Cancer Imaging Program. Her research interests have been both in the role of functional and molecular imaging in the diagnosis and treatment of cancer, as well as evaluating the performance characteristics of imaging modalities for optimal use in the management of the cancer patient. Her work involves establishment of and monitoring of clinical trials to evaluate imaging tracers and techniques, which aim to improve the prevention, diagnosis and treatment of cancer. She provides imaging expertise for trials of cancer diagnostics and therapeutics sponsored by NCI in the clinical trial networks such as NCTN and NCORP. She is the program lead for the Clinical Imaging Steering Committee and The Cancer Imaging Archive. She serves on the RECIST and RANO committees. She has served on FDA’s Medical Imaging Drug Advisory Committee. She serves on the advisory committees of several trans-European biomarker initiatives. She is the recipient of several NIH and NCI Director Awards and is a Fellow of the Society of Nuclear Medicine and Molecular Imaging.
Dr. Shankar received her medical degree in Bangalore University, India and received her M.S. and Ph.D. in Radiation Sciences at Hahnemann University, Philadelphia. She then trained in clinical Nuclear Medicine and completed fellowships in Positron Emission Tomography and Theranostics at the University of Pennsylvania in Philadelphia. Prior to joining the National Cancer Institute, she was a faculty member in the Department of Radiology at Georgetown University and at the Lombardi Cancer Center and also worked in the Division of Nuclear Medicine at Washington Hospital Center.