Vanderbilt University Medical Center
Principal Investigators
Jennifer A. Pietenpol, Ph.D.
Chief Scientific and Strategy Officer
Executive Vice President for Research
Brock Family Directorship in Career Development
Professor of Biochemistry and Otolaryngology
Vanderbilt University Medical Center
1161 21st Ave. S, D-3300 MCN
Nashville, TN 37232
615-936-2033
Ben Ho Park, M.D., Ph.D.
Benjamin F. Byrd Jr. Professor of Oncology
Director, Vanderbilt-Ingram Cancer Center
Professor of Medicine Division of Hematology and Oncology
Vanderbilt University Medical Center
2220 Pierce Ave, PRB 698
Nashville, TN 37232
615-936-1374
Overview
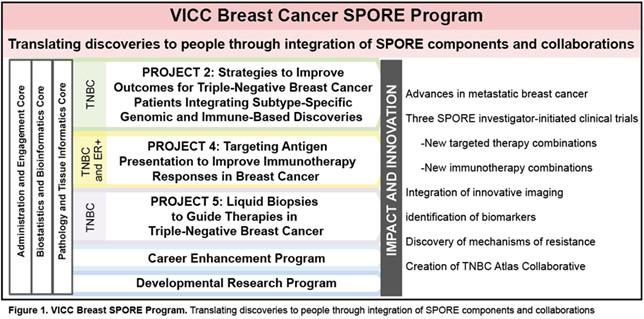
This is the third renewal cycle of the Vanderbilt-Ingram Cancer Center (VICC) Specialized Program of Research Excellence (SPORE) in Breast Cancer, thanks to a very active and strong multidisciplinary VICC Breast Cancer Program that is robustly supported by the SPORE mechanism. Our overall goal remains the same: To conduct collaborative, multidisciplinary and mechanism-based translational research that will have the highest possible impact for women and men with or at risk for breast cancer. After internal and external planning and evaluation, we are conducting three bi-directional translational projects addressing basic and clinical research questions of importance in human breast cancer: Projects 2 and 4 are continuation projects; Project 5 is a new project that has evolved, with co-investigators and co-leaders having previously received Career Enhancement Program (CEP) and Developmental Research Program (DRP) awards in the current and prior funding cycles. Our highly used Pathology and Tissue Informatics (PTIC), Biostatistics and Bioinformatics (BBC) and Administrative and Engagement Cores (AEC) give key support to SPORE investigators (Fig. 1). Three investigator-initiated trials are associated with the highly translational projects in this proposal, all based on extensive collaboration between world-class basic and clinical investigators. The Vanderbilt SPORE team continues to have productive translational collaborations with other Breast Cancer Programs, SPOREs, and national and international groups.
Project 2: Strategies to Improve Outcomes for Patients with Triple-Negative Breast Cancer that Integrate Subtype-Specific Genomic and Immune-Based Discoveries
Project Co-Leaders
- Vandana G. Abramson, M.D. (Clinical Co-leader)
- Jennifer A. Pietenpol, Ph.D. (Basic Co-leader)
Specific Aims
- Aim 1: To determine mechanisms by which standard chemotherapies modulate tumor immune response in combination with checkpoint immunotherapy.
- Aim 2: To determine mechanisms of immune modulation in metastatic TNBC subtypes.
- Aim 3: To evaluate the anti-tumor activity of AR agonists in LAR tumor organoids and identify LAR tumor-specific epithelial genes for diagnostic gene panel development.
Human health challenge: TNBC is one of the most aggressive subtypes, accounting for 25% of all breast cancer deaths. Unfortunately, developing new TNBC treatments has remained challenging; current options are primarily limited to chemotherapy and immunotherapy. Based on preclinical and clinical findings from SPORE-supported NCT03206203 and NCT02457910 (current project period), in the current year, the team will address an unmet medical need: the lack of effective treatment options for individuals with TNBC of the luminal androgen receptor (LAR) subtype.
Translational impact: Using a discovery framework of preclinical models, genomic tools and TNBC patient tumor biospecimens from previous and ongoing Breast SPORE-supported clinical trials, the Project 2 team aims to achieve improved outcomes for LAR subtype [defined as >80% of tumor androgen receptor (AR)+ by immunohistochemistry (IHC)] TNBC patients. Given the dependency of preclinical TNBC LAR models and AR+ TNBC patient tumors on AR signaling, the team hypothesizes that LAR TNBC patients can benefit from AR inhibition in combination with SOC therapy; and that comprehensive molecular analysis of patients’ tumors and ctDNA can provide biomarkers of response and primary resistance. Exploring new treatment combinations is warranted and important, given the lack of effective therapies for this population of breast cancer patients. Through comprehensive molecular and spatial analysis of biospecimens, the established multi-disciplinary team will evaluate the combination of an AR antagonist with SOC agents, develop biomarkers for AR inhibitor response and define the molecular and genetic properties of distinct cell populations that are sensitive or resistant to AR inhibitor therapy.
Project 4: Targeting Antigen Presentation to Improve Immunotherapy Responses in Breast Cancer
Project Co-Leaders
- Laura Kennedy, M.D., Ph.D. (Basic Science Co-leader)
- Justin M. Balko, PharmD, Ph.D. (Clinical Co-leader)
Specific Aims
- Aim 1: Complete correlative analysis of TBCRC043
- Aim 2: Develop cell line and animal model tools for study of specific MHC-I gene loss
The Project 4 team showed that MEK activation suppresses tumor cell surface MHC class I and II and PD-L1 expression in breast cancer. Conversely, inhibition of MEK enhanced these factors, blocked naïve CD8+ T-cell priming, and protected CD8+TIL cytotoxicity by preventing apoptosis driven by chronic TCR stimulation (Clin Cancer Res, 2015 PMC4794351). In luminal-like breast cancer models (MMTV-neu and MMTV-PyVmT), a combination of MEK inhibition and PD-1/PD-L1 inhibitors induced profound anti-tumor effects. Based on these preliminary data, we initiated two clinical trials testing this hypothesis. One of these clinical trials, along with its correlatives, has been submitted to npj Breast Cancer. The other, a much larger study, will complete accrual this year and we are actively generating correlative data from the trial in preparation. This trial was funded by the BCRF, a private foundation, and is entitled “Immunotherapy combination strategies to treat TNBC: a multicenter, multi-arm Translational Breast Cancer Research Consortium (TBCRC) study”. This is a multicenter, open-label, four-arm phase II trial that will evaluate the anti-tumor effect of a PD-L1 inhibitor alone or in combination with either a MEK inhibitor, chemotherapy, or a antibody-drug-conjugate sacizumab govitecan in patients with metastatic TNBC. These trials will test for synergy between MEK inhibition and anti-PD-L1 therapy, identify predictive biomarkers, as well as validate the clinical utility of new therapeutic combinations that promote anti-tumor immunity through enhancement of antigen presentation. Moreover, we are actively expanded preliminary data on loss of individual MHC-I alleles in breast cancer based on striking novel discoveries from our group (CIR, 2025) and from these trials, preparing new models that can be used to evaluate the findings in the laboratory.
Project 5: Liquid Biopsies to Guide Therapies in Triple-Negative Breast Cancer
Project Co-Leaders
- Vandana G. Abramson, M.D. (Clinical Co-leader)
- Ben Ho Park, M.D., Ph.D. (Basic Co-leader)
Specific Aims
- Aim 1: To determine whether patients with metastatic triple-negative breast cancer (mTNBC) who undergo treatment changes guided by ctDNA dynamics demonstrate improved PFS/OS compared to control patients assessed conventionally with imaging alone.
- Aim 2: To validate and functionally assess CHIP mutations as a predictive/prognostic marker for poor response to therapies and worse PFS/OS in patients with metastatic TNBC.
Project 5 will evaluate the clinical utility of using cell-free DNA (cfDNA) in metastatic triple-negative breast cancer (TNBC) patients to guide clinical decision-making. In aim 1, Project 5 will conduct a randomized control trial to determine if instituting second-line therapy with sacituzumab earlier than the first restaging scan can afford better outcomes in TNBC. If it is determined first line chemotherapy is ineffective by measuring quantitative levels of circulating tumor DNA (ctDNA) before and after the initial chemotherapy dose, sacituzumab will be introduced earlier. In aim 2, Project 5 will determine whether clonal hematopoiesis of indeterminant potential (CHIP) mutations found in cfDNA correlate with worse outcomes in patients on our study. Blood cells with and without CHIP mutations will be analyzed from patient samples and assessed on their effects within different subtypes of TNBC cell lines using ex vivo assays. The results of this aim will identify new potential targets (CHIP clones) that could positively affect outcomes for patients with metastatic TNBC.
Administrative Core
Core Co-Directors
Specific objectives: The Administrative Core (AEC) of the VICC Breast SPORE functions as the organizational nexus for all SPORE activities. It aims to address any operational challenges, capitalize on opportunities, and set clear goals for advancing the Breast SPORE. The AEC accomplishes this through the key priorities, including innovation and growth, collaboration and partnerships to share resources, knowledge, and enhancement of research outcomes, talent development through oversight of the DRP and CEP, supportive implementation planning for project and core integration in collaboration with the SPORE IAB and EAB; and performance measurement by establishing clear metrics, evaluation methods, and mitigation strategies, if necessary. The SPORE team will continue to have productive translational collaborations with other Breast Cancer Programs, SPOREs, and national and international groups.
Biostatistics and Bioinformatics Core
Core Co-Directors
Specific objectives: The Biostatistics and Bioinformatics Core supports all VICC Breast SPORE projects, with development of experimental designs, power analysis and sample size estimation, data quality control, statistical/bioinformatics analysis and interpretation of findings, and collaboration on presentation of results.
Pathology and Tissue Informatics Core
Core Co-Directors
Specific objectives: Clinical trial-related tissue collection and curation, procurement for the Vanderbilt Breast Tissue and Body Fluids Repository, provision of specialized technical and diagnostic histopathology services, informatics support (de-identification, histopathological and clinical annotation, tracking and distribution of breast tissues) for translational research by SPORE investigators, linking specimen-specific information to immunohistochemistry (IHC), multiplex immunofluorescence (mxIF), fluorescence in situ hybridization (FISH) assays, and Next Generation Sequencing (NGS) data permitting queries to inform future investigations. Pathology assessments will be made by research and licensed clinical pathologists of the PTIC for clinical/correlative studies within all SPORE clinical trials. The PTIC will also manage the Breast SPORE TNBC Collaborative Atlas.
Developmental Research Program
Program Co-Directors
During the current grant cycle, the SPORE Developmental Research Program awarded 12 pilot projects to 12 investigators. Nine are from four primary departments in the Vanderbilt School of Medicine (Departments Biochemistry, Medicine, Molecular Physiology and Biophysics, and Radiology), two are from departments in the Vanderbilt School of Engineering, two are from the University of Texas Southwestern, and one is from Meharry Medical College. Additionally, seven of the eight research programs at VICC (Breast Cancer, Cancer Cell Biology, Cancer Epidemiology, Cancer Health Outcomes and Control, Genome Maintenance, Host-Tumor Interactions, and Translational Research and Interventional Oncology). Twenty-eight publications have resulted from DRP awardees. Five of the awardees, (Drs. Chazin, Chen, Malladi, Spalluto and Whitehurst) have obtained extramural grants for a combined budget of over $7.1M (direct costs) and $11.6M (total costs). This return in extramural grant dollars far surpasses the investment made in the DRP. Finally, all awardees have presented their research at SPORE monthly meetings and/or the VICC Breast Cancer Program and SPORE Monthly Series.
Career Enhancement Program
Program Co-Directors
During the current project period, the SPORE CEP supported 10 investigators: seven physician-scientists and three basic scientists from three Departments in the School of Medicine at Vanderbilt (Radiology and Radiological Sciences, Medicine, and Biochemistry), the Department of Biochemistry and Cancer Biology at Meharry Medical College and four research programs at VICC (Genome Maintenance, Translational Research and Interventional Oncology, Host-Tumor Interactions and Breast Cancer). Twenty-four publications have resulted from CEP awardees. Six of the awardees, Drs. Dewar, Kennedy, Kim, Philip, Reid, and Sakwe have obtained extramural grants for a combined budget of over $6.7M (direct costs) and $10.3M (total costs). This return in extramural grant dollars far surpasses the investment made in the CEP. In addition, two awardees, Drs. Reid and Kennedy are the clinical co-leaders of Projects 1 and 3, respectively, in the SPORE competing application.