The University of North Carolina at Chapel Hill
Principal Investigators
Victoria Bae-Jump M.D., Ph.D.
Professor, Gynecologic Oncology, School of Medicine
Director, Lineberger Endometrial Cancer Center of Excellence
University of North Carolina at Chapel Hill
Physicians Office Building, Room B105, Division of Gynecologic Oncology, Campus Box 7572, Chapel Hill, North Carolina 27599-7572
(919) 843-4899
Hazel Nichols Ph.D.
Professor of Epidemiology,
Gillings School of Global University of North Carolina at Chapel Hill Public Health
McGavran Greenberg Building 2104F, Campus Box 7435, Chapel Hill, North Carolina 27599
(919) 966-7456
Overview
The Cancer Health Disparities SPORE in Endometrial Cancer is comprised of a multi-disciplinary translational research team and a comprehensive approach to studying disparities in EC that incorporates both measurable social and biological factors into the study design of each of our three Projects and makes use of three unique EC patient cohorts as well as novel pre-clinical mouse and organoid models. The overall mission of our SPORE is to define key biological factors alongside measurable social factors that may drive more aggressive behavior or lead to worse outcomes (treatment response, survival) with each project having a specific focus:
Project 1: Epigenetic aging
Project 2: Microbiome & innate immunity
Project 3: Metabolic reprogramming & allostatic load
Our hypothesis is that social and biological factors are fundamental drivers of cancer health disparities for women with EC, including the inter-related biological factors of epigenetic age acceleration, microbial dysbiosis, a heightened innate immune system, metabolic reprogramming and increased allostatic load.
Specific Aims
Aim 1: Delineate the interplay of measurable social and novel biological factors that contribute to poor outcomes (recurrence, survival, treatment response rate) for EC patients, including epigenetic age (Project 1), the tumor/gut microbiome and innate immunity (Project 2), and metabolic reprogramming and allostatic load (Project 3).
Aim 2: Develop innovative biomarkers related to the disproportionate risk of aggressive EC as well as decreased treatment response and worse survival that span a spectrum of contributing biological pathways (genome/epigenome, microbiome, immune and metabolic).
Aim 3: Identify key molecular, epigenomic, microbial, immune and metabolic targets for therapeutic and preventative interventions (drugs, diet, etc.), specifically tailored to improving care and outcomes for EC patients.
Aim 4: Recruit and support new investigators in EC research through the Career Enhancement Program (CEP) and Developmental Research Program (DRP).
Aim 5: Facilitate high levels of intra-SPORE, inter-SPORE, regional and national collaborations, and relationships with industry and cooperative groups to culminate in the implementation of innovative trials and to facilitate wide-spread sharing of ideas with researchers in the field of EC.
Project 1: Social-environmental measures, epigenetic age, and endometrial cancer disparities
Project Co-Leaders
Ganga Bey, Ph.D., M.P.H. (Applied Co-Leader)
Rebecca Fry, Ph.D. (Basic Co-Leader)
Endometrial cancer (EC) is the 4th most common cancer in US women. EC is plagued by a significant cancer health disparity in survival outcomes between Black and White persons; Black women show a two-fold higher incidence of mortality from this cancer. Several putative causes for this disparity have been proposed including a higher risk for more lethal tumor histologies (serous) and molecular subtypes (TP53 mutations) and greater rates of obesity and diabetes. However, these features alone do not account for the full disparity in EC outcomes, suggesting additional factors are involved. We anticipate that measurable social and environmental factors that have been linked to poorer health outcomes account for a significant proportion of EC survival disparities. Specifically, we anticipate that social and environmental exposures act through epigenetic age acceleration to influence endometrial tumor characteristics, and that individual modifiable psychosocial characteristics may influence these relationships. Our overarching hypothesis is that exposure to measurable social and environmental factors increases likelihood of accelerated biological aging, that individual modifiable psychosocial characteristics differentially influence pace of aging, and that accelerated aging will be associated with tumor characteristics and EC outcome. To understand the relationships among DNAm and cancer health disparities in EC, we will identify the global and site-specific differences in DNAm from ECs from Black and White women in the CECS (Carolina Endometrial Cancer Study), a novel North Carolina-based population-based resource. Our results may improve outcomes for EC patients by leading to the identification of modifiable factors that reduce cancer risk.
Specific Aims
Aim 1. Investigate the associations among measurable social and environmental factors and tumor-based epigenetic age among Black and White EC subjects.
Aim 2. Investigate the association between measurable psychosocial factors and tumor-based epigenetic age among Black and White EC subjects.
Aim 3. Examine whether tumor-based epigenetic age predicts EC tumor characteristics, recurrence, and survival in Black and White EC subjects.
Project 2: Inter-relationship of disparities, the microbiome, innate immune system, and immunotherapy response in endometrial cancer
Project Co-Leaders
Victoria Bae-Jump, M.D., Ph.D. (Applied Co-Leader)
Temitope Keku, Ph.D., M.S.P.H. (Basic Co-Leader)
William Zamboni, Pharm.D., Ph.D. (Basic Co-Leader)
Immune checkpoint blockade targeting programmed cell death protein 1 (PD-1) provides significant clinical benefit in patients with EC. Two interrelated factors that may impact immunotherapy (IO) benefit are (1) the gut and tumoral microbiome, and (2) the innate immune system (IIS) which is responsible for uptake and clearance of monoclonal antibodies (mAbs), such as PD-1 inhibitors. We found that distinct microbiota profiles differentiated ECs of Black vs White women. Black patients have a heightened IIS, which may result in lower exposures of mAbs due to enhanced clearance of the mAbs by the IIS; thus, Black women may need higher doses of PD-1 inhibitors to achieve the same optimal therapeutic response as White women. We hypothesize that differences exist in the tumor/gut microbiome and the IIS of Black women that will culminate in lower IO response and worse survival. Using an ongoing North Carolina population-based study (Carolina Endometrial Cancer Study [CECS]) and a prospective study of UNC EC patients undergoing PD-1 inhibitor therapy (PROMOTE), we will evaluate the EC tumoral and gut microbiota and the IIS in Black and White patients in relationship to measurable social factors, treatment response and survival outcomes. We will also perform more mechanistic studies in a unique germ-free EC mouse model studying fecal microbial transplant (FMT) as a means to improve PD-1 inhibitor treatment response. Ultimately this work will identify future actionable microbial and immune factors and targets for improving disparities in EC to be explored in subsequent clinical trials.
Specific Aims
Aim 1: Delineate differences in the tumor microbiota and the IIS between Black and White women in CECS.
Aim 2: Determine the association between the EC tumor/gut microbiota and the IIS and response of Black vs White women undergoing standard of care PD-1 inhibitor therapy.
Aim 3: Determine if fecal microbial transplants (FMT) from Black vs White EC patients who are resistant vs sensitive to PD-1 inhibitor treatment will impact immuno-responsiveness in germ-free (GF) Lkb1fl/flp53fl/fl mice.
Project 3: Obesity-related metabolic reprogramming in endometrial cancer disparities
Project Co-Leaders
John A. Batsis, M.D. (Clinical Co-Leader)
Melinda Yates, Ph.D. (Basic Co-Leader)
Black women experience the highest increases in EC rates and worse outcomes across all stages and grades. Obesity is tightly linked with EC risk, but decades of research on obesity have yielded modest insight into the specific mechanisms driving EC development, especially in understanding disparities. Roughly 40% of women in the US are classified as having obesity, but only a small fraction of these women develops EC.
Heterogeneity in systemic and tissue-level metabolic disruptions related to obesity and cumulative physiologic stressors may be a key factor for EC risk and contribute to aggressive tumor development. Black women experience higher rates of obesity and type 2 diabetes, higher systemic inflammation, and increased rates of other cardiometabolic risk factors compared to White women. We propose that measurable social and environmental conditions contribute to chronic metabolic and pro-inflammatory states that disrupt endometrial signaling and stress responses. Allostatic load has been used to assess cumulative physiologic stress and is scored using markers of metabolic, cardiovascular, and inflammatory stressors. Allostatic load is associated with morbidity and mortality in aging-related disease, cardiovascular disease, and cancer. At the cellular level, mitochondrial allostatic load describes cumulative stressors or exposures disrupting mitochondrial metabolism and cellular signaling. Our overall hypothesis is that endometrial adaptations to metabolic disruptions in obesity and physiologic stress increase tumorigenesis and promote aggressive tumor behavior underlying EC disparities. Long-term goals are to identify biomarkers for improved EC risk stratification and candidate targets for cancer interception to eliminate EC disparities. We propose to use multiple approaches to investigate cumulative stressors at the systemic, tissue, and cellular (mitochondrial) levels to define pro-tumorigenic signaling.
Specific Aims
Aim 1. Compare cumulative systemic measures of metabolic disruption and physiologic stress (allostatic load) across Black and White women with EC versus women with benign endometrium.
Aim 2. Define endometrial tissue-level reprogramming based on systemic metabolic and physiologic stress to identify regional pathway alterations underlying disparities for Black women.
Aim 3. Define the impact of disrupted mitochondrial function on endometrial stress responses and aggressive tumor behavior using patient-derived organoid models.
Administrative Core
Core Co-Directors
Victoria Bae-Jump, M.D., Ph.D.
Hazel Nichols, Ph.D.
The Administrative Core will serve as the central coordination hub for the Cancer Health Disparities SPORE in Endometrial Cancer investigators and staff, with responsibility for monitoring the progress of all Projects, Cores, and Programs towards translational/ clinical endpoints. The Core will ensure compliance with all University, federal and specific NIH grant regulations and requirements, including timeline communications and consultation with the National Cancer Institute (NCI).
Specific Aims
Aim 1. Provide scientific and administrative oversight for all aspects of the SPORE including fiscal management of NIH funds and the substantial Lineberger Comprehensive Cancer Center institutional commitment.
Aim 2. Organize meetings and manage communications for the Executive Committee, Internal Advisory Board (IAB), External Advisory Board (EAB), and Community Advisory Board (CAB).
Aim 3. Serve as the liaison between the SPORE, UNC and the Lineberger Comprehensive Cancer Center, and NIH/NCI initiatives.
Aim 4. Monitor and support evaluation activities for existing projects and promising developmental research and Career Enhancement Program (CEP) investigators.
Aim 5. Facilitate horizontal (outside the SPORE) and vertical (extensions along the translational continuum) research collaboration, development and implementation.
Biospecimen and Pathology Core
Core Co-Directors
Russell Broaddus, M.D., Ph.D.
Adam Pfefferle, Ph.D.
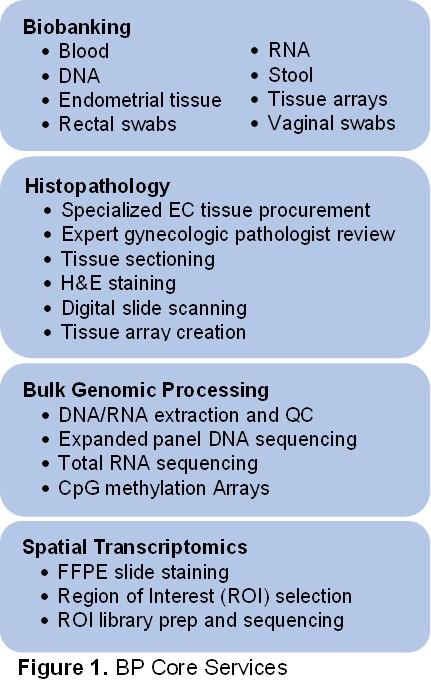
Integrated genomic characterization of endometrial cancer (EC) has significantly contributed to our understanding of the molecular and genetic basis underlying this cancer. A central focus of the SPORE in EC is to integrate these molecular analyses into population-based studies with clinical and epidemiologic annotations. Such correlative studies require three core components for efficient and scalable implementation: 1) a robust biospecimens resource enabling specimen procurement and processing, central pathology review, and clinical data annotation; 2) a coordinated system to facilitate the flow of specimens and data; and 3) highly-automated platforms to support library preparation, sequencing, and other molecular assays. The Biospecimens and Pathology Core will build upon existing infrastructure encompassing these three core components.
Specific Aims
Aim 1. Provide biobanking expertise, coordination, and honest broker support for endometrial tissue, blood, and other SPORE biospecimens
Aim 2. Provide central histopathological review of EC specimens
Aim 3. Perform DNA and RNA genomic assays from bulk EC specimens
Aim 4. Perform spatial transcriptomic assays of EC specimens
Biostatistics and Bioinformatics Core
Core Co-Directors
David Corcoran, Ph.D. (Bioinformatics)
Feng-Chang Lin, Ph.D. (Biostatistics)
The collection and analysis of multi-domain, e.g., social, clinical, and molecular (i.e., high throughput multi-omics), data is needed to comprehensively understand and reduce disparities among endometrial cancer patients. This integration requires the coordination of data across a wide range of statistical, informatics, and clinical expertise. The services provided by the Biostatistics and Bioinformatics (BIOS) Core will expand upon existing resources including the high-performance computer clusters, dedicated storage, standardized data processing workflows, sample tracking, database design, implementation, documentation, maintenance, and user support. The core will ensure all code and data are deposited in public repositories upon completion of each project. Statistical and analytic support will cover the use of field-standard and cutting-edge regression and machine learning approaches for cross-platform and multi-domain data integration.
Specific Aims
Aim 1. Provide biostatistical expertise on methodological aspects of study design.
Aim 2. Update and maintain next-generation sequencing, methylation array, and spatial transcriptomic analysis pipelines.
Aim 3. Provide custom biostatistics and bioinformatics data analysis services for SPORE research projects and teams.
Aim 4. Develop innovative and state-of-the-art analysis methodologies to facilitate translational research activities of the SPORE research teams.
Community Outreach and Engagement Core
Core Co-Directors
Marjory Charlot, M.D., M.P.H., M.S.
Stephanie Wheeler, Ph.D., M.P.H.
Effective community outreach and engagement (COE) is essential for addressing endometrial cancer (EC) disparities. Contributors to EC disparities, broadly and in North Carolina (NC), include disparities in stage of diagnosis, receipt of diagnostic procedures and treatment in compliance with current national guidelines, and aggressive histologies. The COE Core seeks to reduce health disparities in EC by assessing the EC burden and disparities in the NC catchment area, infusing patient and community perspectives into EC research, raising awareness about EC and the importance of biospecimen donation, and disseminating SPORE findings throughout NC and beyond.
Specific Aims
Aim 1: To assess EC burden and disparities in the catchment area, including risk factors, diagnosis and treatment experiences using a novel statewide linked database.
Aim 2: To support the integration of patient and community voices into gynecologic oncology research through expanding partnerships with advocates, healthcare delivery systems, state and community agencies, and coalitions.
Aim 3: To raise awareness about EC and educate communities about the importance of EC biospecimen collection and clinical trials.
Aim 4: To disseminate SPORE findings to audiences in the catchment area and beyond.
Developmental Research Program
Program Director
The Cancer Health Disparities SPORE in Endometrial Cancer Developmental Research Program (DRP) supports innovative, early-phase projects that will provide key insights into critical areas of health disparities in endometrial cancer (EC) research. DRP projects with compelling potential to develop into full SPORE or other NIH-, industry-, or foundation-funded projects will be prioritized. The DRP core will collaborate with the other Cores to connect DRP investigators with relevant expertise, workshops, and collaborative meetings to maximize research potential.
Specific Aims
Aim 1. Stimulate interdisciplinary translational research in Cancer Health Disparities and EC through annual RFA solicitation.
Aim 2. With the Administrative and CEP Cores, review and select two 50K Developmental Research Projects for funding. Unfunded projects will be connected with UNC and SPORE professional development resources and encouraged to revise and resubmit applications in a future funding round.
Aim 3. With the Administrative Core, Executive Committee, and Advisory Boards, monitor DRPs for addition or replacement of SPORE projects. The DRP core will also support investigators in developing future non-SPORE collaborations to extend projects along the translational continuum. \
Career Enhancement Program
Program Director
Ashley Leak Bryant, Ph.D., R.N., O.C.N., F.A.A.N.
The Cancer Health Disparities SPORE in Endometrial Cancer Career Enhancement Program (CEP) supports research activities for oncology clinicians, clinician scientists, and basic, translational, and clinical scientists with strong interest in cancer health disparities and endometrial cancer (EC) research. We will recruit faculty with existing EC science expertise or health disparities research expertise that is being newly applied to the endometrial cancer context to expand the field. The CEP will provide a rigorous mentorship program for awardees and ongoing evaluation of their progress. The SPORE and CEP additionally provide fiscal administration, biostatistics and bioinformatics input, biospecimens, genomics and pathology expertise; patient perspective and integration with community outreach activities; mentorship and critical analysis and constructive feedback on progress via advisory board meetings.
Specific Aims
Aim 1: To promote translational research careers of independent endometrial cancer investigators who can apply their knowledge to address cancer health disparities.
Aim 2: To attract scientists in the field of health disparities to focus on endometrial cancer research.
Aim 3: To develop new scientists to reduce cancer health disparities by making advances in the early detection, prevention, and treatment of endometrial cancer.