Baylor College of Medicine
Principal Investigators
Monica Gramatges, MD, PhD
Professor of Pediatrics
Baylor College of Medicine
One Baylor Plaza
Houston, Texas 77030
(832) 824-4678
Philip Lupo, PhD, MPH
Professor of Pediatrics
Emory University
HSRB2-N344, 1760 Haygood Drive NE
Atlanta, Georgia 30322
(404) 727-5016
Karen Rabin, MD, PhD
Professor of Pediatrics Institution
University of California, San Francisco
Helen Diller Family Cancer Research Building,
1450 3rd Street
San Francisco, California 94158
(415) 353-7251
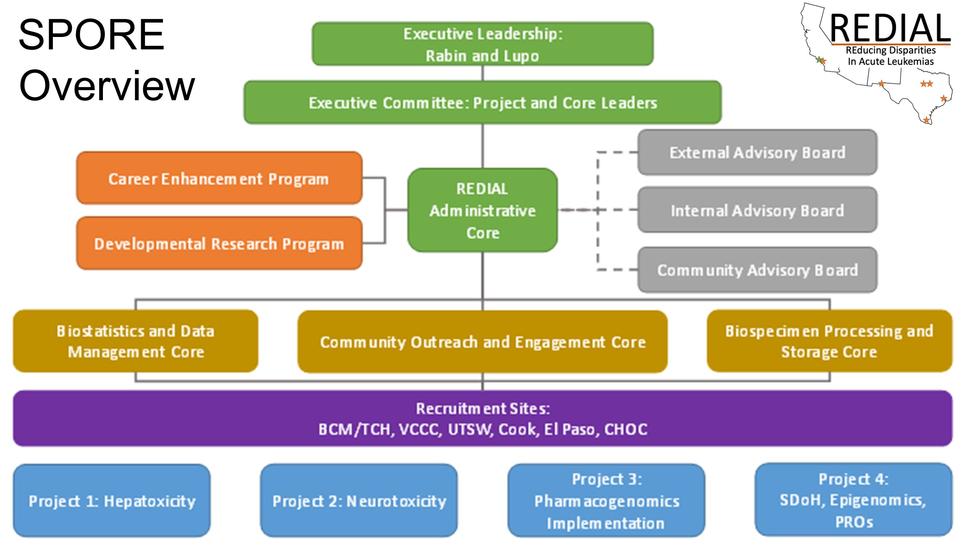
Overview
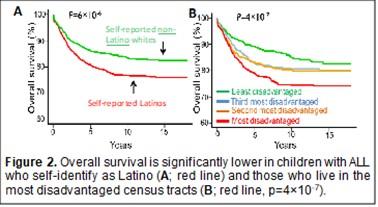
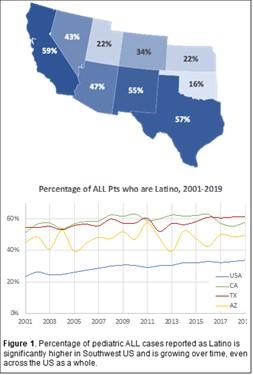
Acute lymphoblastic leukemia (ALL) is the most common cancer in children. Although cure rates have improved over the past 50 years, cancer outcomes differ by population. Latinos have both the highest incidence of leukemia and the lowest survival rates in the U.S. There are many potential reasons for these differences in health outcomes.
The overall goals of this U54 program are to reduce population-based health outcome differences among children and adolescents with ALL by identifying genetic and environmental factors that may have contributed to adverse outcomes. This program is the first Specialized Programs of Research Excellence (SPORE) devoted to pediatric leukemia. The four Research Projects will investigate four areas in which population-based health outcome differences have been observed, including hepatotoxicity (Project 1), neurotoxicity (Project 2); pharmacogenomics (Project 3); and genetic and environmental contributors to patient-reported outcomes (Project 4).
The ultimate goal of this SPORE is to pursue prevention and treatment strategies aimed at reducing cancer outcome differences in all these areas. The Program will be administered through the Administrative Core (Core A). Biospecimens will be processed by Core B, and statistical analysis will be provided by Core C. Core D will facilitate community outreach and engagement. A Developmental Research Program will foster development of innovative pilot projects that aim to understand and/or reduce health outcome differences in children with ALL, and a Career Enhancement Program will recruit, train, and guide physicians and scientists to become successful translational investigators focusing on population-based health outcome differences in pediatric ALL.
We will leverage the REducing Disparities in Acute Leukemia (REDIAL) Consortium, comprising 6 cancer centers in the southwestern U.S., to generate a rich dataset derived from over 7,000 children and adolescents with newly diagnosed ALL and a biorepository of bone marrow, blood, buccal cells, and cerebrospinal fluid samples from nearly 2,000 subjects. This invaluable resource will also serve as an important asset for current and future investigations of factors associated with health outcome differences in ALL. This work will form the foundation for developing effective risk prediction and intervention strategies to reduce population-based health outcome differences in children and adolescents with ALL.
Project 1: Understanding Population-Based Disparities in Treatment-Associated Hepatotoxicity During ALL Therapy
Project 1 will integrate clinical, demographic, lipidomic, and genomic data to advance our understanding of health outcome differences in liver toxicity (hepatotoxicity) early in leukemia treatment. Lipidomics refers to the study of lipid (fat) molecules in the body including the types of lipids present and how they are metabolized and stored.
Improved treatment for pediatric ALL has resulted in over 90% survival, but nearly a quarter of patients experience treatment-associated hepatotoxicity (TAH). The Project 1 team, co-led by Drs. Austin Brown (BCM), Van Huynh (UC Irvine), Etan Orgel (Keck School of Medicine) and Steve Mittelman (David Geffen School of Medicine), has identified striking population-based differences in the incidence of TAH during the initial induction phase of ALL therapy. A significant difference is Latino children are more likely to develop TAH that requires lowering the dose of chemotherapy, potentially impacting cancer treatment efficacy and leading to higher relapse and poorer survival.
This project explores how differences in inherited and other factors contribute to the development of TAH. Our team has developed a model to predict risk for TAH and has found that abnormal lipids may be a biomarker for TAH risk. This project will also use mouse models of ALL to understand the etiology of TAH.
Our Aims are to examine:
- If changes in plasma lipid profiles related to liver function contribute to TAH during pediatric ALL induction therapy
- How clinical, demographic, genetic, and lipidomic data can inform the prediction of TAH in pediatric ALL
- How genetic variation and dyslipidemia contribute to TAH using a mouse model of ALL
The research outlined in this proposal will address key gaps in our understanding of TAH as a cancer health outcome and is expected to support future research endeavors. Ultimately, we anticipate that this line of research will inform risk-stratified approaches to safely deliver curative cancer treatment to children and adolescents treated for ALL and improve their overall survival.
Project 2: Understanding Population-Based Disparities in Methotrexate Neurotoxicity and Its Impact on Short- and Long-Term Adverse Outcomes Among Children and Adolescents with Acute Lymphoblastic Leukemia
The antifolate agent methotrexate (MTX) is a critical component of curative pediatric ALL protocols. All pediatric ALL patients receive MTX in some form, whether oral, intravenous (IV), or with a lumbar puncture (intrathecal, or IT). About 10% of pediatric ALL patients experience neurotoxicity following IT or high-dose IV MTX. However, Latino children appear to experience MTX-associated neurotoxicity more frequently. The clinical management of MTX-related neurotoxicity often involves treatment delays and/or modifications, which may limit treatment efficacy and impact survival.
A number of factors may contribute to differences in pediatric ALL neurotoxicity and related treatment outcomes, including clinical characteristics, disease features, and other factors. Some of these and other differences observed in pediatric ALL outcomes can be explained by genetic differences, suggesting a role for MTX drug metabolism underlying risk for MTX-associated neurotoxicity. Our goal is to understand the factors contributing to differences in treatment-related toxicities and health outcomes among children and adolescents with ALL.
Project 2, co-led by Dr. Michael Scheurer, Dr. Karen Rabin, and Dr. Austin Brown will investigate how genetics, tumor biology, and environmental factors contribute to poorer outcomes in at-risk populations. Our Aims are to:
- Test a risk prediction model for acute MTX neurotoxicity among children with ALL
- Determine the impact of acute MTX neurotoxicity on ALL relapse
- Determine the impact of acute MTX neurotoxicity on long-term neurocognitive problems
This project will provide insights into the factors responsible for increased neurotoxicity and subsequent increased risk of relapse and long-term neurocognitive deficits in specific high-risk populations of children with ALL.
Project 3: Pharmacogenomic Advancements for Improved Treatment of Children and Adolescents with Acute Lymphoblastic Leukemia
Genetic variation has the potential to influence the risk of ALL treatment-related toxicity. There are known changes in certain genes that play a role in chemotherapy metabolism that increase a patient s risk for side effects, such as prolonged time to blood count recovery after treatment, neuropathy, and anemia. Therefore, it is important to test for these genetic changes and, when present, use this information to guide cancer treatment and clinical care. However, key implementation gaps remain, including an insufficient understanding of how frequently these genetic changes occur in populations such as Latinos and a limited understanding for how to best communicate these genetic test results and related recommendations to patients and families.
Project 3, co-led by Drs. Brooke Bernhardt, Stacey Pereira, Hadley Stevens Smith, and Philip Lupo, will investigate if Latino children experience more treatment-related toxicity with ALL treatment than non-Latinos, and if there is a role for genetic testing-informed individualized cancer treatment to reduce the risk for adverse outcomes.
Our Aims are to:
- Implement a clinical genetic testing panel in a large population of ALL patients and test clinical and family-centered outcomes
- Identify Latino-specific genetic variation in ALL drug-gene pairs that are associated with treatment-related toxicities
This project will comprehensively address the implementation of genetic testing to assess patient metabolism of drugs used for ALL treatment, and is expected to identify new, Latino-specific genetic changes that will inform risk stratification strategies and genetic testing protocols among children and adolescents diagnosed with ALL.
Project 4: Patient-Reported Outcomes, Epigenetic Profiles, and Neighborhood Social Determinants of Health in Childhood Acute Lymphoblastic Leukemia (ALL)
Health outcome disparities have multifactorial root causes, such as differences in disease biology, host factors related to cancer susceptibility and response to treatment, or exposure to a broad spectrum of environmental factors that impact health. These differences, including both individual and neighborhood factors as well as factors related to access to care and infrastructure, may disproportionately affect certain groups, particularly non-English speakers, and are major contributors to health risks and outcomes. For example, children with ALL that come from families with lower income, or who live in areas where they are surrounded by people with a similar background, are more likely to relapse and less likely to survive their disease.
Few studies conducted in childhood cancer populations collect information about income and access to resources, and less than 10% measure patient-reported outcomes (PROs). There is newer evidence for a genetic link between childhood neighborhood environment and adult health. This linkage is related to epigenetics, which is a term that describes how genes are expressed or turned on/turned off in response to the environment in which you live. Project 4, co-led by Drs. Monica Gramatges, Melissa Richard, and Amy Hughes, will identify associations between environmental and social factors, PROs, and epigenetic changes in both Latino and non-Latino children with ALL. The team will use these data to develop a psychosocial intervention for at-risk children.
Our Aims will:
- Collect PRO data at three on-treatment time points and in survivorship using validated measures and assess the relationship between social factors and PROs in children with ALL
- Evaluate the relationship between social factor-associated epigenetic profiles and PROs in Latino and non-Latino children with ALL
- Use PRO data and interviews with patients and families to develop a psychosocial support intervention for at-risk children with ALL
The team will prospectively assess the feasibility and acceptability of the intervention in the target population, addressing a critical unmet need to evaluate and mitigate health outcome differences in at-risk populations.
Administrative Core
Core A, the Administrative Core, will oversee and coordinate all entities of the SPORE, which includes four Research Projects (Project 1, therapy-associated hepatotoxicity; Project 2, methotrexate neurotoxicity; Project 3, pharmacogenomics; and Project 4, genetic and environmental factors and patient-reported outcomes), three additional Cores (Core B, the Biospecimen Processing and Storage Core; Core C, the Biostatistics and Data Management Core; Core D, a Community Outreach and Engagement Core), a Developmental Research Program (DRP); and a Career Enhancement Program (CEP).
By facilitating employment, training, regulatory compliance, internal and external communication, and financial management of the SPORE, Core A will maximize the productivity and efficiency of all participating components. Under the leadership of Co-Directors Drs. Karen Rabin and Philip Lupo, Core A will be responsible for overseeing the conduct of the research at each site, including the regulatory, financial, and human resources aspects. Core A will also ensure regular internal communication among investigators and sites and organize external communication with leaders in the field, the scientific community, and patient advocacy organizations.
Core A will:
- Oversee all human resource management responsibilities, including creation of job descriptions and the hire, evaluation, and replacement of personnel locally and at the Consortium sites
- Provide regulatory management and governance, including tracking regulatory submissions/approvals and assisting with necessary training of research staff
- Control expenditures and maintain budget information for all Projects, Cores, and Consortium sites
- Facilitate internal and external communication among all participating Project Leaders, Core Directors, and sites to ensure adequate enrollment rates, address logistical issues, and present updates on research progress
Internal, External, and Community Advisory Boards (IAB, EAB, CAB) will be established. Both the IAB and EAB will include leaders in pediatric leukemia biology, genomics, epidemiology, supportive care, and patient advocacy, including two leaders of funded SPOREs. The IAB will meet twice yearly, the EAB annually, and the CAB quarterly.
In summary, the Administrative Core will ensure that all aspects of the proposed research program are conducted in accord with the highest standards of organization, documentation, communication, and fiduciary responsibility.
Biospecimen Processing and Storage Core
The Projects proposed in this application rely on the submission of serial peripheral blood, bone marrow, buccal, and cerebrospinal fluid (CSF) specimens from children and adolescents with Acute Lymphoblastic Leukemia (ALL) across the six participating clinical recruitment sites of the REducing Disparities in Acute Leukemia (REDIAL) Consortium. The Biospecimen Processing and Storage Core (Core B), co-led by Terzah Horton, MD, PhD and Michael Scheurer, PhD, MPH, will serve as the central location where these samples will be processed into the materials needed for the specific studies associated with Research Projects 1 (treatment-associated hepatotoxicity), 2 (methotrexate neurotoxicity), 3 (pharmacogenomics), and 4 (genetic and environmental factors and patient-reported outcomes), as well as determination of genetic ancestry for use across all Projects. In addition, this Core will biobank material that will be available for pilot projects through the Developmental Research Program (DRP) and Career Enhancement Program (CEP). This Core will support the collection, shipping, processing, and storage of patient samples from each study site, as well as provide up-to-date and accurate sample annotation. Core B will follow previously developed standard operating procedures to ensure uniform collection and processing for optimal sample yield and quality.
The Aims of Core B are to:
- Receive and catalog blood, bone marrow, buccal samples, and CSF provided by the Consortium site
- Process all patient samples to isolate plasma, peripheral blood mononuclear cells, and bone marrow mononuclear cells, and separate blasts from non-blasts where appropriate
- Allocate and distribute samples to Projects
- Maintain a biobank of samples for future research
The resulting biobank will be an extremely valuable resource for this U54 translational research grant and future translational studies examining health outcome differences in childhood ALL.
Biostatistics and Data Management Core
The translational research projects proposed in this Specialized Programs of Research Excellence (SPORE) grant application integrate many different types of existing and newly generated data, including biological, clinical, demographic, genomic, metabolomic, lipidomic, molecular, environmental, patient-reported outcomes, and survey and questionnaire data. The Biostatistics and Data Management Core (Core C), co-led by Dr. Hong Zhu PhD from the University of Virginia and Dr. Cristian Coarfa PhD from Baylor College of Medicine, functions as a centralized research design and data science coordination center for all SPORE projects, bringing together expertise and intellectual resources in biostatistics, bioinformatics, geospatial data analysis, and data management for SPORE investigators.
Core C members will:
- Provide biostatistical and bioinformatics support for rigorous research design and state-of-the-art statistical analysis for all SPORE projects
- Facilitate state-of-the-art geospatial data analysis for all SPORE projects
- Develop and maintain an integrated database environment for efficient data management, integration, analysis and sharing for all SPORE research projects, investigators, and participants
Core C members will work collaboratively with investigators to ensure that the appropriate design is incorporated into all studies from inception, that data are appropriately validated, linked, cleaned, and analyzed, and that all results will be interpreted for statistical as well as biological and clinical significance.
The Biostatistics and Data Management Core will work with the Administrative Core (Core A) to assist in preparation of reports, data management and other administrative duties, with the Biospecimen Processing and Storage Core (Core B) for management and analysis of specimen-related data, and with Community Outreach and Engagement Core (Core D) for providing relevant community engagement documents. Fully integrating Core C members into all SPORE projects and other Cores will build workflow efficiencies and support the translational goals of the SPORE, while enhancing quality and ensuring scientific integrity of the participating projects. Core C will provide sophisticated data management, design, and analysis services in order to facilitate important discoveries about ALL health outcomes and to guide future prevention and intervention strategies.
Community Outreach and Engagement Core
The Community Outreach and Engagement (COE) Core, co-led by Dr. Sandi Pruitt PhD at University of Texas Southwestern and Dr. Ashley Butler PhD at Baylor College of Medicine (BCM), will facilitate partnerships among SPORE investigators and community partners within the Texas and California catchment area.
The Core D Aims are to:
- Develop infrastructure and provide technical support to ensure study procedures are patient-centered
- Facilitate bidirectional communication between investigators and communities, including children and adolescents and their caregivers
The COE Core will provide training and education to investigators and community partners; facilitate convening of a Community Advisory Board (CAB) and listening sessions with community members; provide customized Stakeholder Engagement Studios for SPORE research; and serve as the nexus for information learned through COE activities and dissemination of scientific findings to communities within the catchment area. The COE Core of the SPORE will provide a model of infrastructure and engagement activities for community engagement in health outcomes research in pediatric cancer.
Dissemination of lessons learned to the pediatric cancer research community will provide an important framework for engaging population-based communities in rigorous and community-informed research aimed toward eliminating health outcome differences in at-risk populations.
Developmental Research Program
The objective of the Developmental Research Program (DRP) of this SPORE, co-led by Drs. Monica Gramatges and Brooke Bernhardt, is to support the development and successful completion of innovative, high-risk/high-reward pilot projects that aim to understand or reduce health outcome differences in childhood Acute Lymphoblastic Leukemia (ALL). Drs. Gramatges and Bernhardt have strong track records in translational research investigating clinical, genetic, and molecular predictors of treatment-related toxicities in childhood leukemia.
DRP-supported projects may be basic, clinical, or translational but must address population-based differences in ALL treatment-related toxicities (acute or late onset), or outcomes in relation to social determinants of health. Selection for funding will be determined by the DRP Committee, with representation across all participating sites to ensure transparency in award distribution. Committee members include faculty with expertise in clinical and translational research and a strong track record of participation in grant peer review, as well as members of the Community Advocate Board. Criteria for funding will be work with strong scientific merit in line with the SPORE objectives and with strong potential for future funding. Proposals representing collaborations between participating institutions will also be prioritized.
In addition to grant review and selection, the DRP Committee will be responsible for monitoring and oversight of funded projects via regular review of progress and final reports.
The DRP's core mission is to provide grant awardees with:
- Funding support
- Access to Core resources
- Mentorship during the award period
For each award year $100,000 is budgeted to support funding two new or one new/one renewal award. All Pilot Grant PIs will have access to U54 Core Facilities at no cost. To conduct their proposed projects, applicants may utilize either REDIAL data and biospecimens or external data sources. The DRP Committee will assign each funded project an Advisory Committee with relevant expertise, who will provide mentorship and guidance specific to each project. A key role of the Advisory Committee, together with the DRP Committee, will be to facilitate the translation of DRP-funded projects to work that is included in a U54 SPORE project or that is independently funded.
Career Enhancement Program
Translational research related to population differences in pediatric Acute Lymphoblastic Leukemia (ALL) is lacking, with a shortage of investigators investing their career in this area. The Career Enhancement Program (CEP) is designed to provide incentives, training and guidance to attract academic physician-scientists, clinician-investigators, and laboratory-based scientists to dedicate their efforts to this area of research. A key goal of the CEP is to support early faculty in order to nurture a robust future translational research environment. Another goal is to support established investigators who may wish to refocus their careers on research in this area.
To meet these goals, the specific aims of the CEP are to recruit, train, and guide a new group of physicians and scientists to become successful translational investigators focusing on population-based disparities in outcomes in pediatric ALL; to educate awardees in the basic principles of ALL biology, epidemiology, therapy and toxicities, clinical outcomes, and the multifactorial bases for population-based disparities in outcomes; and to develop and collect metrics to assess the success of the CEP, allowing the program to be reviewed and modified as necessary. To achieve these aims, we have developed specific criteria for selection and funding through a peer-reviewed mechanism, and we will collaborate with the Baylor College of Medicine Office of Community Engagement in the selection of candidates for the CEP program.
The SPORE will also take advantage of best practices and evaluation tools identified in other centers and entities at BCM that have pilot project funding. Strong mentorship is an important component of the CEP, through which awardees will be instructed in the principles of clinical, basic, and translational research relating to ALL outcome disparities. Population-based mentors will provide role models to CEP awardees. Specific areas of education include scientific and clinical methods, biomedical ethics, statistical design and analysis, genetics and genomics, pharmacology, epidemiology, and areas relevant to individual projects. Mentorship will include laboratory-based investigators, clinical investigators, biostatisticians and epidemiologists. Mentors from within the U54 SPORE as well as other faculty members at BCM and the other SPORE sites will provide the expertise required for the development of strong, productive translational research skills.