What are biospecimens?
Biospecimens are biological materials from people (such as tissue, blood, plasma, and urine) that can be used for diagnosis and research. Biospecimens, sometimes called samples, may contain DNA, RNA, proteins, and other molecules important for understanding disease. They help to tell the story of your cancer.
Medical information is often requested from patients to go along with their biospecimen donation. This makes donated biospecimens even more helpful for research. Information could include medical records that patients give consent to provide, as well as records from clinical trials that patients volunteer to join.
Federal guidelines put a high priority on protecting the privacy of personal and medical information.
Are patients with cancer required to donate biospecimens for research?
Patients are not required to donate biospecimens. However, many patients have given consent for their biospecimens to be used in research, in the hope that the resulting knowledge might help other patients in future years.
What are biorepositories (biobanks)?
Biorepositories are "libraries" where biospecimens are carefully stored. Biorepositories are critical for enabling modern molecular-based research to improve clinical outcomes for patients. Biospecimens are made available to qualified scientists to study for clinical or research purposes.
How can I donate biospecimens?
Biospecimens become available for medical research when patients donate tissue or blood during surgery, biopsy, blood draw, or other medical procedures. Learn more about biospecimen donation.
How are biospecimens used in research?
Scientists have developed many ways to identify genes and proteins, learn about the role they play in the origin and progression of disease, and link a patient's genes to their response to drugs and medicines. These advances are sometimes known as molecular medicine.
In addition to looking at molecular information, scientists analyze a vast amount of clinical information about patients' health and disease. Information from patient records and clinical trials helps the scientists identify patterns and disease sub-types. This leads to strategies for diagnosing and treating cancer in new and more effective ways.
Human biospecimens can provide a bridge between emerging molecular information and clinical information. Researchers use specimens to isolate the molecular characteristics of actual human disease and correlate those patterns with what is known about how people experience that disease (the disease's clinical progression).
Human biospecimens can be used to:
- Identify and validate ways to deliver drugs or agents to specific cells
- Identify how diseases progress and vary
- Group patients as more or less likely to respond to specific drugs
- Group patients to determine which treatment is appropriate
- Develop screening tests to detect biomarkers that are associated with certain stages or sub-types of a disease
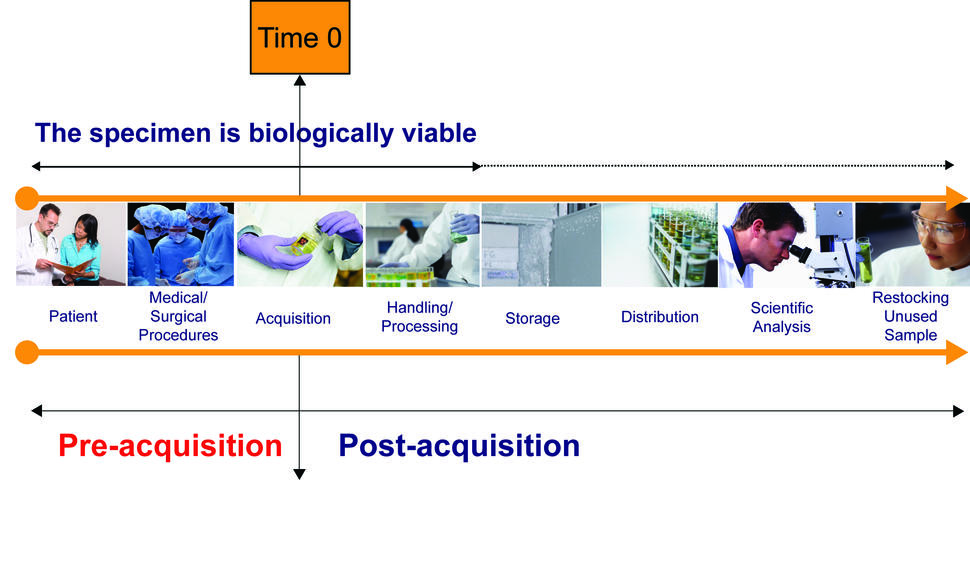
Lifecycle of Biospecimens
Patient: The "specimen" exists in situ within the specific biologic context of the individual patient.
Medical/Surgical Procedures: Examples include administration of antibiotics, anesthesia, and other drugs, disruption of blood supply to the tissue (arterial clamping intra-operatively), systemic blood pressure variations during surgery, intra-operative blood loss, or intra-operative administration of blood, blood products or other fluids (hemodilution). At this point, anesthesiologists, nurses, or surgeons may introduce variables into the procedure that change the biologic context of the "specimen" and, as a result, cause dynamic changes in the molecular profiles within the "specimen".
Acquisition: Various methods of acquiring the specimen may introduce specific types of stress or trauma for the living specimen. Nurses handling the specimen in the operating room and delivering it to the pathologist as well as the clerks and pathologists receiving the specimen may all introduce time delays or other variables such as packaging in plastic bags (changing exposure to desiccation or oxidation) in the acquisition process.
Handling/Processing: Examples of preparation variables introduced prior to and during biologic stabilization of the specimen include time at room temperature, temperature of room, type of fixative, time in fixative, method and rate of freezing, and size of specimen aliquots. Pathologists, pathology assistants, technicians, or pathology residents in training may contribute to the variation during this phase.
Storage: The storage temperature and duration of storage may impact specimen quality. Progressive dehydration, desiccation, or oxidation may occur.
Distribution: Variations in transport conditions may cause alterations in the specimen. The people who package the specimens, those who transport the specimens and those who receive and deliver the samples to individual investigators may all contribute to variation at this point.
Scientific Analysis: Different methods of analysis for various classes of biomolecules may be variably affected by any of the factors above. Even with a single analytic method, each individual investigator and technician involved in the specimen analysis may contribute to analysis variation.
Restocking Unused Sample: Environmental variables of unpredictable types may be introduced during the return of unused specimen samples from individual research laboratories to storage.