NCI-led Studies Crucial for FDA Approval of Selumetinib for Adults with Neurofibromatosis Type 1 (NF1) with Symptomatic Inoperable Plexiform Neurofibromas (PN) Diseases
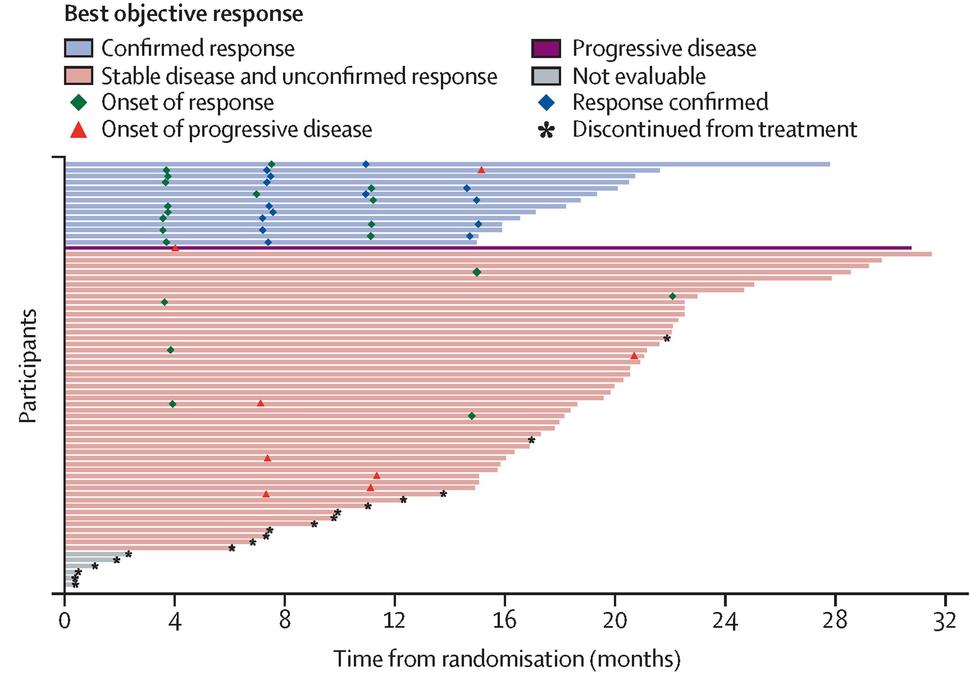
Swimmer plot showing best response* in participants randomly assigned to selumetinib (full analysis set)
Credit: Chen et al., 2025 (Lancet, Figure 2A)
The FDA recently approved selumetinib, an oral kinase inhibitor, to treat adults (18 years or older) with the rare NF1 with symptomatic inoperable PN diseases.
Dr. Alice Chen, head of NCI’s Developmental Therapeutics Clinic (DTC), co-led the international randomized, double-blind placebo phase 3 KOMET trial with additional DTC staff that contributed substantially to selumetinib’s approval in adult patients. DTC and NCI’s Pediatric Oncology Branch (POB) staff provided guidance on the development of KOMET.
KOMET showed significant objective response rate (ORR) of 20% (n=14/71; 95% CI 11.2 to 30.9) by cycle 16 versus people on placebo with 5% ORR (n=4/74; 1.5 to 13.3) reported in Lancet.
The international approval of selumetinib in both adults and children was driven by work from the POB and DTC, as previously highlighted in the NEJM and Nature Medicine publications. This serves as a strong example of the transformative work the NCI can deliver in the rare tumor field.
Learn more about the KOMET clinical trial results published in The Lancet.